Multiple myeloma (MM) is a common hematologic malignancy with variable degrees of immunodeficiency. Disease- and treatment-related compromise of the immune system predisposes patients to infections, which are a major cause of morbidity and mortality.
ObjectiveWe aimed to establish the incidence and main characteristics of infections in MM patients treated at our center over a 10-year period.
Method and resultsOf the 412 patients retrospectively analyzed, 154 (37.4%) were documented to have at least one episode of infection and were included in this study. A total of 244 infectious episodes were documented. The most common site of infection was the lung, followed by the genitourinary system. The most common infections were bacterial, followed by viral. Escherichia coli were the most common organism. In 160 (65.5%) episodes, the organism was not isolated. Thalidomide with dexamethasone was the most common treatment regimen, followed by melphalan with dexamethasone. Infection was the main cause of death in 26 (6.3%) out of all 412 patients.
ConclusionInfections are a notable cause of morbidity and mortality in the clinical course of MM patients. By considering patient and disease characteristics, a risk-adapted selection of the MM treatment should be employed, with special attention toward patient age and disease-associated organ dysfunction. Patient education, access to healthcare and physician vigilance are also essential. Vaccination and antimicrobial prophylaxis may be considered prior to or during therapy.
Multiple myeloma (MM) is characterized by the malignant proliferation of plasma cells, and variable degrees of immunodeficiency have been associated with the disease.1 Overproduction of monoclonal, non-functional paraprotein and suppression of polyclonal B lymphocytes by malignant plasma cells result in hypogammaglobulinemia. Infiltration of the bone marrow crowds out normal hematopoiesis, reduces the number of functional leukocytes and causes defects in cell-mediated immunity by suppressing T lymphocytes and natural killer cells.2,3 Recently, patients with monoclonal gammopathy of undetermined significance have been noted to have an increased risk of infections, corroborating the role of plasma cell dyscrasias with immunodeficiency.4 In addition, organ damage related to disease and therapy-related adverse effects also increase susceptibility to infections.5,6
Multiple myeloma is considered a disease of advanced age (median age at diagnosis ∼70 years), with several patients having pre-existing comorbidities, which further increases the risk of mortality from infections.7 Development of new chemotherapeutic agents and treatment strategies have significantly improved the survival of MM patients over the past few decades, transforming MM into a chronic condition, one with multiple relapses and the need for subsequent salvage therapies, which further decrease cell-mediated immunity.8–10 Hence, it is important to manage complications of the disease and its treatment as patients live longer.
Herein, we describe the frequency and characteristics of infections in MM patients treated at our institution over a 10-year period.
MethodsA retrospective chart review was conducted for patients diagnosed with MM and/or treated with conventional chemotherapy for MM over a 10-year period (from 2008 to 2017) at the Aga Khan University Hospital, Karachi, Pakistan. Since this was a retrospective chart review, informed consent was waived and ethical exemption was obtained from the Ethics Review Committee at the Aga Khan University Hospital. Patients were identified using the International Classification of Diseases coding and their charts were reviewed. Diagnosis of MM was made based on raised monoclonal gammopathy with an ‘M’ component, percentage of plasma cells in the bone marrow (10–30%) and/or evidence of raised serum calcium level, renal dysfunction, anemia and lytic bone lesions on skeletal survey (CRAB) criteria. The diagnosis of infection was made based on clinical signs and symptoms, positive microbiological cultures and/or positive radiological findings indicative of infection in the precise clinical context according to standard practice.11 Diagnosis of fungal urinary tract infection (UTI) was made using clinical signs/symptoms of UTI with significant pyuria, but no bacteria on urinalysis or bacterial growth on urine culture, and positive fungal growth in urine culture. All patients with at least one episode of infection during the course of their treatment were included in this study.
Information was written on a semi-structured questionnaire and consisted of demographic and basic patient characteristics, clinical and laboratorial characteristics of MM and chemotherapy regimens. Information on the details of each infectious episode, including site and infectious organism, was also collected. Any and all information gathered from the medical records of patients for the purpose of this study was kept confidential and disclosed only to the primary team conducting the study. Lastly, the outcome of these patients was analyzed. All data were entered into the Statistical Package for the Social Sciences (SPSS) version 19 (SPSS Inc., Chicago, IL, USA) to calculate descriptive statistics, standard deviations and range of all variables.
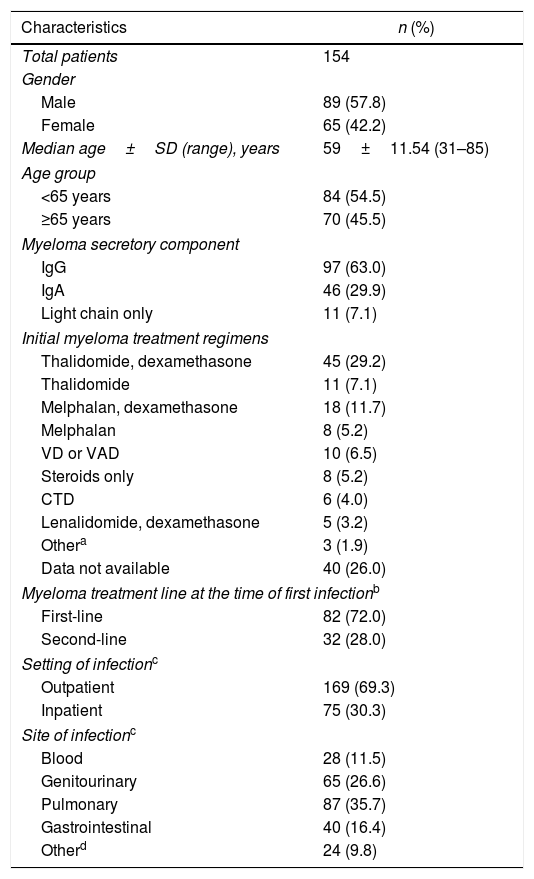
ResultsDuring the study period, a total of 412 patients were diagnosed and/or treated for MM at the Aga Khan University Hospital using conventional chemotherapy. Patients receiving high-dose chemotherapy with stem cell transplantation were not included. One hundred and fifty four (37.4%) patients were documented as having had at least one episode of infection during the conventional chemotherapy treatment (which excluded high-dose chemotherapy with stem cell transplantation) and were included in the study. The baseline details of these 154 patients are displayed in Table 1. There were 89 males and 65 females. The median age±SD was 59±11.54 years. The most frequent initial clinical presentation of MM was anemia, seen in 101 patients (66%). This was followed by hypercalcemia, in 49 patients (30%), and renal failure (serum creatinine ≥2.0mg/dL), in 42 patients (27%). The IgG paraprotein was the most common secretory component. The skeletal survey was normal in 38 patients (24.7%). Sixteen patients (10.4%) had one to two lytic lesions at presentation, while 52 patients (33.7%) had three or more lesions. The skeletal survey was not available for 48 patients. Bone marrow biopsy results were available for 63 patients, all showing plasmacytosis of ≥10% (range: 10–90%).
Baseline characteristics of study population.
| Characteristics | n (%) |
|---|---|
| Total patients | 154 |
| Gender | |
| Male | 89 (57.8) |
| Female | 65 (42.2) |
| Median age±SD (range), years | 59±11.54 (31–85) |
| Age group | |
| <65 years | 84 (54.5) |
| ≥65 years | 70 (45.5) |
| Myeloma secretory component | |
| IgG | 97 (63.0) |
| IgA | 46 (29.9) |
| Light chain only | 11 (7.1) |
| Initial myeloma treatment regimens | |
| Thalidomide, dexamethasone | 45 (29.2) |
| Thalidomide | 11 (7.1) |
| Melphalan, dexamethasone | 18 (11.7) |
| Melphalan | 8 (5.2) |
| VD or VAD | 10 (6.5) |
| Steroids only | 8 (5.2) |
| CTD | 6 (4.0) |
| Lenalidomide, dexamethasone | 5 (3.2) |
| Othera | 3 (1.9) |
| Data not available | 40 (26.0) |
| Myeloma treatment line at the time of first infectionb | |
| First-line | 82 (72.0) |
| Second-line | 32 (28.0) |
| Setting of infectionc | |
| Outpatient | 169 (69.3) |
| Inpatient | 75 (30.3) |
| Site of infectionc | |
| Blood | 28 (11.5) |
| Genitourinary | 65 (26.6) |
| Pulmonary | 87 (35.7) |
| Gastrointestinal | 40 (16.4) |
| Otherd | 24 (9.8) |
VD: vincristine, dexamethasone; VAD: vincristine, doxorubicin, dexamethasone; CTD: cyclophosphamide, thalidomide, dexamethasone.
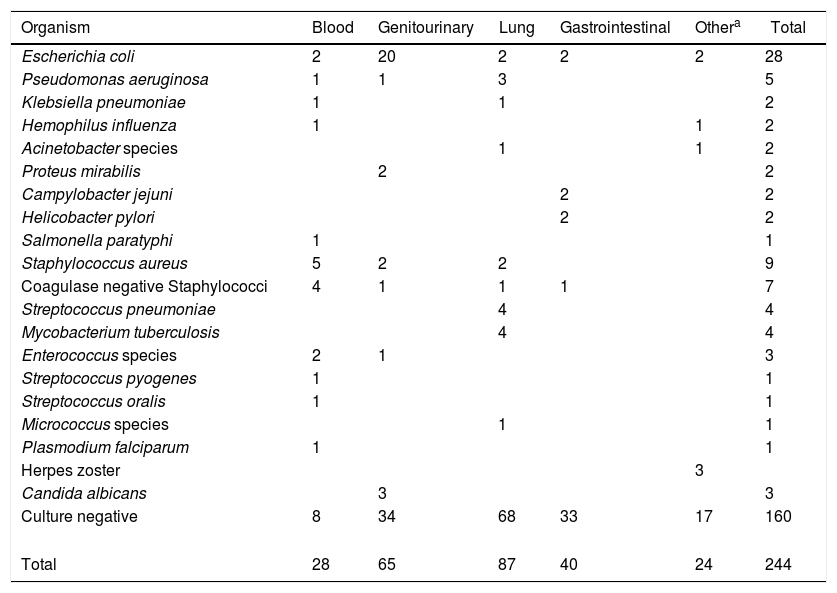
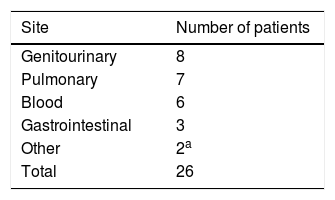
A total of 244 infectious episodes were documented, out of which 169 (69.3%) were in the outpatient setting, while 75 (30.7%) were in the inpatient setting. The most common site of infection was the lung, followed by the genitourinary system (Table 1). The most common infections were bacterial, followed by viral. One hundred and sixty (65.5%) infectious episodes were culture negative. Escherichia coli were the most commonly isolated infectious organisms in 28 episodes (11.5%). Three patients were diagnosed with fungal UTIs with Candida albicans (two patients during the first-line treatment with thalidomide and dexamethasone and one patient during the second-line treatment with cyclophosphamide monotherapy). The complete details of infectious organisms according to the infection sites are given in Table 2. Of the infectious episodes with positive cultures, 45 infections were attributed to gram negative bacteria, whereas 30 infections were due to gram positive bacteria. The median duration of the follow-up was 28 months (range 2–97 months), calculated from the date of the MM diagnosis to the date of the last follow-up. Out of the total of 412 MM patients during the 10-year period, 26 (6.3%) died of infections (Table 3).
Details of 244 infectious episodes in patients with multiple myeloma.
| Organism | Blood | Genitourinary | Lung | Gastrointestinal | Othera | Total |
|---|---|---|---|---|---|---|
| Escherichia coli | 2 | 20 | 2 | 2 | 2 | 28 |
| Pseudomonas aeruginosa | 1 | 1 | 3 | 5 | ||
| Klebsiella pneumoniae | 1 | 1 | 2 | |||
| Hemophilus influenza | 1 | 1 | 2 | |||
| Acinetobacter species | 1 | 1 | 2 | |||
| Proteus mirabilis | 2 | 2 | ||||
| Campylobacter jejuni | 2 | 2 | ||||
| Helicobacter pylori | 2 | 2 | ||||
| Salmonella paratyphi | 1 | 1 | ||||
| Staphylococcus aureus | 5 | 2 | 2 | 9 | ||
| Coagulase negative Staphylococci | 4 | 1 | 1 | 1 | 7 | |
| Streptococcus pneumoniae | 4 | 4 | ||||
| Mycobacterium tuberculosis | 4 | 4 | ||||
| Enterococcus species | 2 | 1 | 3 | |||
| Streptococcus pyogenes | 1 | 1 | ||||
| Streptococcus oralis | 1 | 1 | ||||
| Micrococcus species | 1 | 1 | ||||
| Plasmodium falciparum | 1 | 1 | ||||
| Herpes zoster | 3 | |||||
| Candida albicans | 3 | 3 | ||||
| Culture negative | 8 | 34 | 68 | 33 | 17 | 160 |
| Total | 28 | 65 | 87 | 40 | 24 | 244 |
Infections directly leading to mortality in 26 patients.
| Site | Number of patients |
|---|---|
| Genitourinary | 8 |
| Pulmonary | 7 |
| Blood | 6 |
| Gastrointestinal | 3 |
| Other | 2a |
| Total | 26 |
The initial treatment regimens were available for only 114 patients. The most frequent treatment regimen was thalidomide and dexamethasone, followed by melphalan and dexamethasone. Eighty-two (72.0%) patients experienced their first episode of infection during the first-line treatment, while the remaining 32 (28.0%) experienced it during the second-line treatment (Table 1). The median time from the initiation of the MM therapy to the first infectious episode was 127 days.
DiscussionInfections are a major cause of morbidity and mortality in patients with MM, severely affecting the ability to treat, treatment outcomes and survival.12 Vulnerability to infection in MM is multifactorial. Key disease-related and therapy-related factors that contribute to increased susceptibility to infections are discussed in detail in previously published articles.5,6
To date, there has been a scarcity of data regarding the spectrum of infectious complications in patients with MM in Pakistan. In our 10-year study period, infections occurred in 154 (37.4%) patients. The most frequently isolated organisms were E. coli, followed by Staphylococcus aureus, coagulase negative Staphylococci and Pseudomonas aeruginosa. We observed a greater number of bacterial infections attributed to gram-negative bacteria in comparison to gram-positive bacteria, a finding which is concordant with previous data.13 However, some studies have reported gram-positive and gram-negative bacteria to cause almost an equal number of infections in MM.5,14,15
There is a substantial increase in the risk of infection within the first year following diagnosis (11-fold in bacterial and 18-fold in viral infections),10 with the greatest risk of infection being in the first 2 months of starting therapy and in patients with active (particularly relapsed or refractory) disease.13,16 In fact, the MM patient group at highest risk for developing serious infections is comprised of patients with renal failure, with approximately one-third of these patients dying within the first 2 months of diagnosis.17 Most infections in newly diagnosed, treatment-naïve patients and during the first few months of therapy are attributable to Streptococcus pneumoniae,18 although one must note that this observation is reported in older studies. Pneumonia and septic arthritis are the most common infections in this subgroup. In fact, infection is often the initial presentation that leads to the work-up and eventual diagnosis of MM in a notable proportion of patients.13,19–21 In contrast, patients who develop renal failure and those with advanced or relapsed/refractory disease suffer from infections mainly due to gram-negative bacilli or S. aureus. Several factors, such as prolonged hospitalization, indwelling catheters and central lines, chemotherapy (especially steroid-based regimens or repeated cycles) and bone marrow infiltration by MM, are implicated in these patients.16,22
Fever in an MM patient due to the disease itself is exceedingly rare23 and almost always indicates infection and therefore should be taken very seriously. A detailed history and physical examination to ascertain the possible site of infection are paramount. Empiric treatment covering both encapsulated and gram-negative bacteria should be started while awaiting identification of causative organism(s) via cultures.13 The choice of antibiotics should always be determined according to the pattern of antibiotic resistance at each institution.
The respiratory tract and genitourinary system were the two most common sites of infection in our study, which is consistent with data from previous reports.10,13,19,20 Although numerous cultures from patients with respiratory tract infections were negative for microorganism growth, S. pneumoniae was the most commonly isolated organism in our study. S. pneumoniae, S. aureus and Hemophilus influenzae are the most common causative organisms of respiratory tract infections in MM.13 As for the genitourinary system, E. coli were the most frequently isolated organisms from our patients. Historically, E. coli and gram-negative species such as Pseudomonas, Proteus and Klebsiella are the main causative organisms of UTIs in patients with MM.13,19
Some studies14,24 report an increased incidence of fungal infections in patients with MM, however, only 3 of our patients suffered from fungal infections. This might be explained by the fact that we excluded all infectious episodes that occurred during and after high-dose chemotherapy and autologous hematopoietic stem cell transplantation, which is complicated by profound neutropenia and mucositis (well-known predisposing factors for fungal infections).25 Similar to our results, Valković et al.,14 reported no fungal infections in their cohort of non-transplanted patients. Earlier studies have also reported a low incidence of fungal infections in patients with MM.19
A notable number of studies discuss the impact of infections on the outcomes of MM patients and mortality. Doughney et al.,19 observed a substantial 26% infectious mortality in their cohort. Perri et al.,16 and Shaikh et al.,24 reported infectious mortality rates of 17.4% and 17.0%, respectively. Another study reported an infection-related death rate of 14.5% in newly diagnosed MM patients undergoing induction chemotherapy.8 In fact, a large study involving 3000 MM patients reported a staggering 45% early mortality rate (within 6 months of diagnosis/therapy) attributable to infections26 and another large study comprised of 9253 patients showed a 22% mortality within the first year of MM diagnosis.10 While these mortality rates are notable, some studies have stated comparatively lower mortality rates due to infection. For example, Valković et al.,14 reported a mortality rate of 9.3% due to infections. Offidani et al.,6 stated that only 1 of 85 (1.1%) MM patients with infections died. A mortality rate of 1.5% was observed in patients with MM in a study by Caravita et al.,27 The mortality rate in the present study due to infections was 6.3%, which is comparable with mortality rates reported in the literature.
Although this is first study from Pakistan investigating the spectrum of infections in patients with MM, our study has limitations due to its retrospective nature. One drawback is a large number of culture negative infectious episodes, which may have led to an underestimation of the frequency of causative organisms and an incomplete characterization of the spectrum of infections in our study population. Since a vast majority of our patients never had their serum beta-2 microglobulin measured (due to financial constraints), staging according to the International Staging System could not be determined and incorporated into our results. Missing patient data, especially urinary light chain excretion, made it difficult to stage patients according to the Durie-Salmon system as an alternative. Another limitation is the lack of a control group, which made it difficult to ascertain disease and patient characteristics that may play important roles as predisposing factors to infections. Prospective studies are recommended, which would effectively eliminate these limitations and provide an in-depth characterization of infectious complications in patients with MM. Furthermore, the inclusion of a control group may also help identify risk-factors associated with infection, which would help direct individualized patient care and treatment strategies.
Prophylactic antibiotics may have a potential role in reducing the incidence of infection in MM patients. A study by Oken et al.,28 showed that antimicrobial prophylaxis with trimethoprim–sulfamethoxazole (TMP–SFX) during the first 2 months of anti-myeloma treatment significantly decreased the risk of bacterial infections (7.1% vs. 42.3% in controls) and infectious mortality (3.6% vs. 15.4% in controls). However, a relatively newer and larger study from 2012 showed no difference in severe bacterial infections between prophylaxis with ciprofloxacin vs. TMP–SFX vs. observation only in the first 2 months of treatment.29 Key concerns with the use of prophylactic antibiotics are the increased risks of developing Clostridium difficile infections and antibiotic resistance, which recommend against their routine use.30
Another plausible strategy to reduce the incidence of infections is to consider preemptive vaccination, especially with killed, component and/or conjugated vaccines. However, since three quarters of MM patients demonstrate suboptimal humoral immune responses and decreased polyclonal immunoglobulin synthesis,31 the feasibility and efficacy of vaccination in this patient population is uncertain. Even in the patients who are able to mount a humoral immune response to vaccination, the antibody response is short-lived. This was shown in a study that reported a decline in antibody titers to pre-immunization levels within 18 months after vaccination with the pneumococcal vaccine.32 One possible strategy is to vaccinate MM patients more frequently than the normal population. Since vaccination is relatively less expensive and less toxic, exploration of its use as a prophylactic measure in future studies is warranted and may yield positive findings.
The first step in managing infections in patients with MM is a risk-adapted selection of the MM treatment which takes under consideration the patient and MM characteristics, with special attention towards patient age and disease associated organ dysfunction. Patient education, 24 hour access to healthcare advice and treatment, and physician vigilance are all essential in preventing and managing infections in patients with MM. The decision to vaccinate and administer antimicrobial prophylaxis should be determined based on these factors, as well as the choice of the anti-myeloma treatment. These measures may lead to individualized preventive measures to reduce infection risk in MM patients while keeping negative consequences, such as the risk of resistance development with prophylactic antibiotic use and adverse reactions to vaccines, to a minimum.
Conflicts of interestThe authors declare no conflicts of interest.