Thrombotic microangiopathies (TMA) are a group of disorders with overlapping clinical features that require urgent intervention. Treatment is based on the recognition of the TMA type, which is often challenging. The aim of this study was to identify specific HLA associations with different TMA types to aid rapid diagnosis and appropriate treatment, since the HLA assay can be completed within five hours.
MethodsAll 86 consecutive patients who presented to the University of Arkansas for Medical Sciences between May 2013 and January 2021 with a presumptive diagnosis of TMA were included in this study. HLA typing was performed and correlated with other clinical and laboratory studies.
ResultsIn comparison with other types of TMA, patients with acquired thrombotic thrombocytopenic purpura (aTTP) showed increased frequencies of HLA-DRB1*11, HLA-DQB1*03:01/19, HLA-DRB1*08 and HLA-DRB3. Combining the presence of these HLA associations with a PLASMIC score of 6 or more achieved a higher positive predictive value (90%) for identifying aTTP than the PLASMIC score alone (69%). In comparison with other TMA types, patients with aTTP showed decreased frequencies of HLA-DRB4, HLA-DRB1*07, HLA-DQB1*02. The HLA-DRB1*07/DQB1*02 was not observed in any aTTP patients (negative predictive value: 100%), and thus the presence of this haplotype essentially rules out aTTP. Further, HLA-DRB1*11/DQB1*03:01/19 was absent in atypical hemolytic uremic syndrome patients.
ConclusionHLA alleles can be used as an adjunct for the rapid assessment of TMA and can help to differentiate it from other primary and secondary forms of TMA, allowing for earlier definitive therapy.
Thrombotic microangiopathies (TMA) are a group of disorders characterized by thrombocytopenia, microangiopathic hemolytic anemia and microvascular occlusion resulting in end-organ ischemia and infarction.1 These disorders can be broadly categorized into primary and secondary syndromes. Primary syndromes include thrombotic thrombocytopenic purpura (congenital or acquired ADAMTS13 deficiency [aTTP]), shiga toxin-related hemolytic uremic syndrome (HUS) or complement-mediated HUS, also called atypical HUS (aHUS). Secondary TMA can be due to a variety of causes such as malignant hypertension, autoimmune diseases (systemic lupus erythematosus [SLE], scleroderma, catastrophic antiphospholipid antibody syndrome), disseminated intravascular coagulation (DIC), infections, sepsis, solid organ or hematopoietic stem cell transplantation, medications (e.g., cyclosporine, ticlopidine, clopidogrel, quinine), pregnancy associated (HELLP syndrome), and others.
Although TMA are rare (e.g., the incidence of aTTP in adults is 3 per 1,000,0002 and incidence of HUS in children is 3 per 100,0003) they require urgent intervention based on recognition of the underlying pathophysiology. However, diagnosing the specific cause is often challenging as these conditions have overlapping clinical features. The diagnosis requires an extensive work-up that can include stool culture or polymerase chain reaction (PCR) for shiga toxin gene detection (for HUS), complement levels and mutations involving complement factors (for aHUS), ADAMTS13 activity and antibodies/inhibitor levels (for aTTP), along with other tests including complete blood count with peripheral blood smear examination, lactate dehydrogenase (LDH), serum haptoglobin, liver function tests, renal function tests, antinuclear antibody (ANA), lupus anticoagulants, antiphospholipid antibodies, and viral serology among other things. Some of these assays, particularly ADAMTS13 and complement mutation analysis, are not available at all facilities, and thus samples are often sent to reference laboratories, making them unsuitable for real-time clinical decision making at most institutions.4
Given the high mortality rate for aTTP, treatment (therapeutic plasma exchange (TPE) and/or caplacizumab) is usually initiated urgently based on a high index of clinical suspicion, even without ADAMTS13 activity results; currently no gold standard for the diagnosis of aTTP is available. Recent studies have utilized the PLASMIC scoring system for rapid assessment of adults with TMA to predict the likelihood of aTTP.5 In a meta-analysis, Paydary et al.6 confirmed the diagnostic accuracy of the PLASMIC score (99% sensitivity, 57% specificity, and 99% negative predictive value [NPV]), while other studies have not found the PLASMIC score a reliable tool for establishing, or not establishing, a diagnosis of aTTP.7,8
Prior studies have shown increased susceptibility for aTTP with the presence of HLA-DRB1*11, DQB1*03:01, and DQB1*02:02, while the presence of HLA-DRB4 and HLA-DRB1*04 have been found to be protective.9-12 However, no studies have been performed to look for HLA associations with other causes of TMA.
HLA typing has a fast turnaround time and results can be available in less than five hours. Many facilities that perform TPE also have an associated HLA laboratory that routinely performs HLA testing for deceased organ donors or patients with platelet refractoriness during after-hours and on weekends. By comparison, ADAMTS13 testing is less commonly available in-house even at larger facilities and University hospitals and is often sent out to reference laboratories. Hence, HLA typing might be useful as an adjunct test, if available, to aid in differential diagnosis of TMA. The objective of this study was to look for associations between HLA and various causes of TMA, and to use this information to initiate specific therapy in a timelier manner.
Patients and methodsThis study was approved by the Institutional Review Board at University of Arkansas for Medical Sciences (UAMS). All 86 consecutive patients with suspected TMA between May 2013 and January 2021 were included in the study. These patients typically presented to the emergency room or clinical services with one or more symptoms including fever, neurological impairment, renal impairment and gastrointestinal symptoms. A provisional diagnosis of TMA was based on the presence of thrombocytopenia and microangiopathic hemolytic anemia at initial work-up (complete blood count and peripheral blood smear). Once a provisional diagnosis of TMA was made by the clinical team, transfusion medicine specialists were consulted to perform TPE for possible aTTP while waiting for additional testing results including ADAMTS13 activity and inhibitor levels, complement levels and mutations involving complement factors (aHUS genetic panel), DIC panel, serum haptoglobin, comprehensive metabolic panel, liver function tests, ANA, lupus anticoagulant panel, antiphospholipid antibodies, viral serology. Ethical approval was obtained from the institutional review board to perform HLA typing on blood obtained for routine clinical care after all diagnostic testing had been performed. HLA typing was performed using either rSSO-PCR (LabType®,OneLambda®) or NexGen Sequencing (TruSight® HLA v2, Illumina®) or both. PLASMIC score was calculated based on the study by Bendalapudi et al. (2017).5
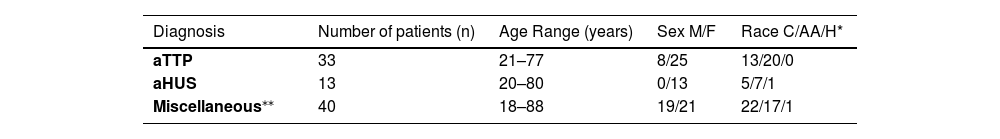
While information and testing were gathered prospectively, the overall data analysis was performed retrospectively. Patients were sorted into three groups based on laboratory results. Patients with low ADAMTS13 activity (≤10) and presence of ADAMTS13 inhibitor were grouped as aTTP (33 patients) based on the International Society on Thrombosis and Haemostasis (ISTH) guidelines.13 Patients with complement factor mutations were grouped as aHUS (13 patients). The remaining patients were grouped as miscellaneous (secondary TMA, 40 patients) and included patients with SLE, scleroderma, dermatomyositis, sepsis, DIC, underlying malignancy, end stage renal disease, malignant hypertension, drug-induced TMA, HELLP syndrome, deceased donor transplant recipients, and hematopoietic cell transplant recipients. The study population is shown in Table 1.
Characteristics of 86 patients presenting with suspected thrombotic microangiopathies.
| Diagnosis | Number of patients (n) | Age Range (years) | Sex M/F | Race C/AA/H* |
|---|---|---|---|---|
| aTTP | 33 | 21–77 | 8/25 | 13/20/0 |
| aHUS | 13 | 20–80 | 0/13 | 5/7/1 |
| Miscellaneous⁎⁎ | 40 | 18–88 | 19/21 | 22/17/1 |
C: Caucasian; AA: African American; H: Hispanic; aTTP: acquired thrombotic thrombocytopenic purpura; aHUS: atypical hemolytic uremic syndrome.
Miscellaneous: systemic lupus erythematosus (SLE - 8), disseminated intravascular coagulation/sepsis (10), hypertension (4), end stage renal disease (2), deceased donor kidney transplant (2), hematopoietic stem cell transplant (1), malignancy (2), dermatomyositis (1), scleroderma (2), drug-induced (tacrolimus [1], carfilzomib [1]), multifactorial (2), and HELLP syndrome (4).
The HLA allele frequencies (observed and expected) were determined for all patients in each TMA group. Briefly, the observed allele frequency was the actual number of occurrences of the specific allele in the current study population. The expected HLA frequencies based on the race (African Americans and Caucasian) were obtained as reported by the United Network for Organ Sharing (UNOS) (https://unos.org/wp-content/uploads/CPRA_frequencies.xls). The UNOS data are felt to accurately reflect the distributions of HLA types in the US population based on race/ethnicity. However, the racial/ethnic distribution of the current cohort varies in each TMA category and differs significantly from those of the general US population and shows an over-representation of African Americans (Supplemental Table 1). Additionally, African American patients are highly overrepresented in aTTP cohorts in the United States.14 Therefore, the expected allele frequencies for all patients in each TMA category were adjusted to accommodate the differences between this patient population and the general US population by race/ethnicity.15 Briefly, the expected allele frequency for a particular HLA allele was calculated by adjusting the known race/ethnic frequency of the specific HLA allele with the race/ethnic frequency found in the population of this study. The observed HLA allele frequencies were then compared with the expected HLA allele frequencies as percentages for each group. The observed versus expected allele frequencies for individual races in each group were also compared to determine the impact of individual races on disease association. Chi-square analyses were performed for samples with larger numbers (>20), and Fisher Exact analyses were performed with smaller sample sizes (≤ 20). The p-values were calculated using Microsoft Excel®, and although a p-value <0.05 is considered clinically significant, a more stringent p-value (≤0.01) was used for determining an association with specific HLA types.
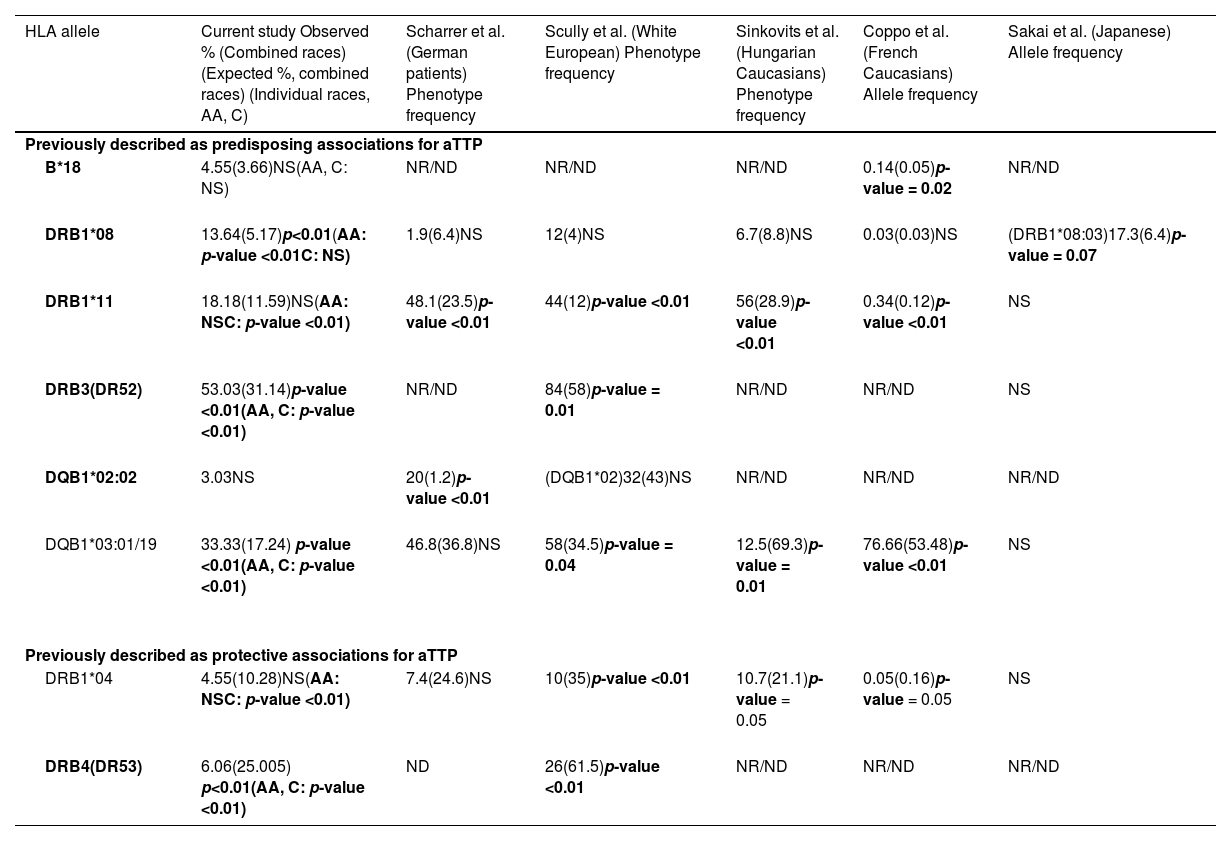
ResultsAllele frequenciesTable 2 summarizes the observed versus expected allele frequency percentages in the different TMA groups. Table 3 compares the allele frequency percentages noted in the current study to previously published reports on HLA-associations in aTTP patients.9-12,16.
Observed versus Expected allele frequencies in the different thrombotic microangiopathies categories.
| HLA allele | aTTP Observed % (Expected %) p-value | aHUS Observed % (Expected %) p-value | Miscellaneous Observed % (Expected %) p-value |
|---|---|---|---|
| Previously described as predisposing associations for aTTP | |||
| DRB1*08(Combined races) | 13.64(5.17)p-value <0.01 | 7.69(4.94)NS | 3.75(8.59)NS |
| AA | 20(6.59)p-value <0.01 | 0(6.59)NS | 5.88(6.59)NS |
| C | 3.85(2.98)NS | 14.29(2.98)NS | 0(2.98)NS |
| DRB1*11(Combined races) | 18.18(11.59)NS(p-value = 0.05)1 | 0(10.76)NS | 6.25(12.76)NS |
| AA | 12.5(13.03)NS | 0(13.03)NS | 5.88(13.03)NS |
| C | 26.92(9.36)p-value <0.01 | 0(9.36)NS | 6.82(9.36)NS |
| DRB32(DR52) | 53.03(31.14)p-value <0.01 | 34.62(31.14)NS | 51.25(31.14)p-value <0.01 |
| DQB1*03:01/19(Combined races) | 33.33(17.24%)p-value <0.01 | 0(17.63)NS(p-value = 0.02)1 | 15.00(22.91)NS |
| AA | 35(16.93)p-value <0.01 | 0(16.93)NS | 17.65(16.93)NS |
| C | 30.77(17.71)p-value <0.01 | 0(17.71)NS | 11.36(17.71)NS |
| B*18(Combined races) | 4.55(3.66)NS | 3.85(3.87)NS | 5.00(4.15)NS |
| AA | 2.50(2.72)NS | 0(2.72)NS | 2.94(2.72)NS |
| C | 7.69(5.11)NS | 7.14(5.11)NS | 6.82(5.11)NS |
| DQB1*02:023 | 3.03 | 15.38 | 3.75 |
| Previously described as protective association for aTTP | |||
| DRB1*04(Combined races) | 4.55(10.28)NS | 7.69(13.2)NS | 7.5(16.23)NS |
| AA | 5(5.69)NS | 0(5.69)NS | 5.88(5.69)NS |
| C | 3.85(17.33)p-value <0.01 | 7.14(17.33)NS | 11.36(17.71)NS |
| DRB42(DR53) | 6.06(25)p-value <0.01 | 23.08(25)NS | 15(25)NS |
aTTP: acquired thrombotic thrombocytopenic purpura; aHUS: atypical hemolytic uremic syndrome; NS: Not significant; AA: African American; C: Caucasian.
Expected allele frequencies for AA and C reflect those reported by the United Network for Organ Sharing (UNOS) (https://unos.org/wp-content/uploads/CPRA_frequencies.xls). Expected allele frequencies for combined races in each group were calculated by adjusting the known race/ethnic frequency of the specific HLA allele with the frequency found in the current study population. The observed allele frequency was the actual number of occurrences of the specific allele in this study population.
Results did not reach clinical significance based on our stringent criteria of p-value ≤0.01, but showed a trend with a p-value <0.05.
Specific breakdown of inheritance patterns of HLA-DRB3, -DRB4, and -DRB5 by race are not available, therefore we cannot assess increased or decreased levels of these by race/ethnicity in our data analyses. HLA-DRB3, -DRB4, and -DRB5 are broadly associated with specific HLA-DRB1 types, which can be more readily associated with specific race/ethnic groups.
Allele frequencies noted in acquired thrombotic thrombocytopenic purpura patients in our study in comparison with the prior reported studies.
aTTP: acquired thrombotic thrombocytopenic purpura; NR/ND: Not reported/Not done.
The frequency of HLA-DRB1*08 was found to be significantly higher in aTTP patients (p-value <0.01). Further analysis based on race showed that frequency was significantly higher in African American patients than in Caucasian patients (p-value <0.01). No such trend was noted in other TMA groups.
A trend in increased frequency of HLA-DRB1*11 in patients with aTTP (p-value = 0.05) was observed. Further analysis based on race showed that the frequency was significantly higher in Caucasian patients but not in African American patients. Thus, given the higher number of African American patients in the population of this study, the overall results only showed a trend and did not reach statistical significance. In contrast, none of the patients with aHUS expressed HLA-DRB1*11. In the miscellaneous category, the observed frequency of HLA-DRB1*11 was not significantly different to the expected frequency, irrespective of the race.
A higher frequency for HLA-DRB3 was noted in aTTP patients (p-value <0.01) and the miscellaneous category (p-value <0.01); statistically significant associations were not observed in patients with aHUS. Specific breakdown of inheritance patterns of HLA-DRB3, -DRB4, and -DRB5 by race are not available, therefore we cannot assess increased or decreased levels of these by race/ethnicity in the data analyses. HLA-DRB3, -DRB4, and -DRB5 are broadly associated with specific HLA-DRB1 types, which can be more readily associated with specific race/ethnic groups.
HLA-DQB1*03:01/19 was significantly increased in patients with aTTP (p-value <0.01) and was found to be statistically significant in both African American and Caucasian patients. Interestingly, none of the patients with aHUS had HLA-DQB1*03:01/19. In the miscellaneous category, the observed frequency of HLA-DQB1*03:01/19 was not significantly different to the expected frequency, irrespective of the race.
A statistically significant increase in the frequency of HLA-B*18 in aTTP patients or in other TMA was not observed. Conversely, an increased frequency for HLA-DQB1*02:02 was observed in patients with aHUS, but no such trend was noted in other TMA. However, specific breakdown of inheritance patterns of HLA-DQB1*02:02 by race are not available, therefore we cannot assess increased or decreased levels of these by race/ethnicity in the data analyses.
Protective allele frequenciesHLA-DRB1*04 and HLA-DRB4 have been reported to be protective alleles in prior studies,10-12 and hence observed at a lower frequency in aTTP patients. As shown in Table 2, HLA-DRB1*04 was present at a lower frequency in these aTTP patients, and significantly reduced in Caucasian patients but not in African American patients. Given the higher number of African American patients in the current study population, the overall results did not reach statistical significance. HLA-DRB4 was also present at a lower frequency in patients with aTTP.
No statistically significant differences were noted for HLA-DRB1*04 and HLA-DRB4 in other TMA groups.
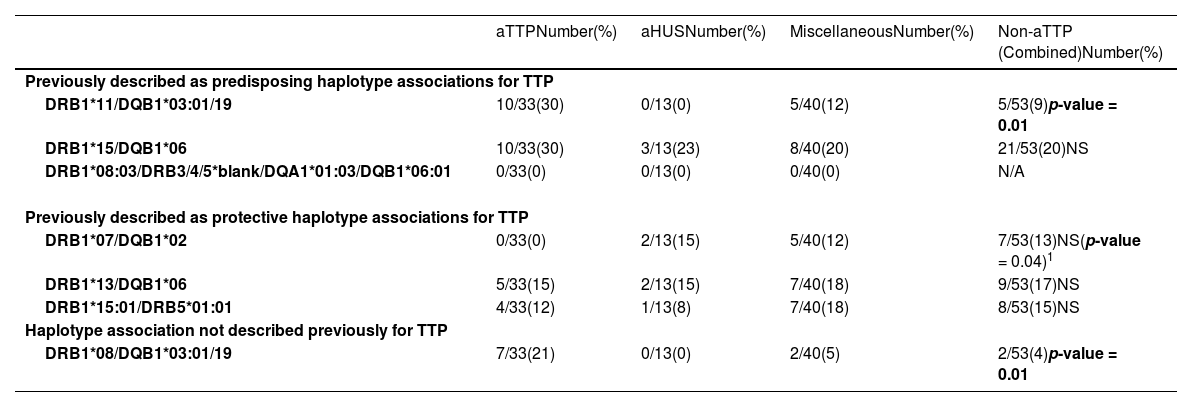
Haplotype frequenciesNext the presence of predisposing and protective HLA haplotype frequencies in the different TMA groups were evaluated (see Table 4).Table 5 compares the haplotype associations found in the current study to previously published reports on HLA haplotype associations in aTTP patients.
Haplotype frequencies in the different thrombotic microangiopathies categories as compared with acquired thrombotic thrombocytopenic purpura.
| aTTPNumber(%) | aHUSNumber(%) | MiscellaneousNumber(%) | Non-aTTP (Combined)Number(%) | |
|---|---|---|---|---|
| Previously described as predisposing haplotype associations for TTP | ||||
| DRB1*11/DQB1*03:01/19 | 10/33(30) | 0/13(0) | 5/40(12) | 5/53(9)p-value = 0.01 |
| DRB1*15/DQB1*06 | 10/33(30) | 3/13(23) | 8/40(20) | 21/53(20)NS |
| DRB1*08:03/DRB3/4/5*blank/DQA1*01:03/DQB1*06:01 | 0/33(0) | 0/13(0) | 0/40(0) | N/A |
| Previously described as protective haplotype associations for TTP | ||||
| DRB1*07/DQB1*02 | 0/33(0) | 2/13(15) | 5/40(12) | 7/53(13)NS(p-value = 0.04)1 |
| DRB1*13/DQB1*06 | 5/33(15) | 2/13(15) | 7/40(18) | 9/53(17)NS |
| DRB1*15:01/DRB5*01:01 | 4/33(12) | 1/13(8) | 7/40(18) | 8/53(15)NS |
| Haplotype association not described previously for TTP | ||||
| DRB1*08/DQB1*03:01/19 | 7/33(21) | 0/13(0) | 2/40(5) | 2/53(4)p-value = 0.01 |
aTTP: acquired thrombotic thrombocytopenic purpura; aHUS: atypical hemolytic uremic syndrome;.
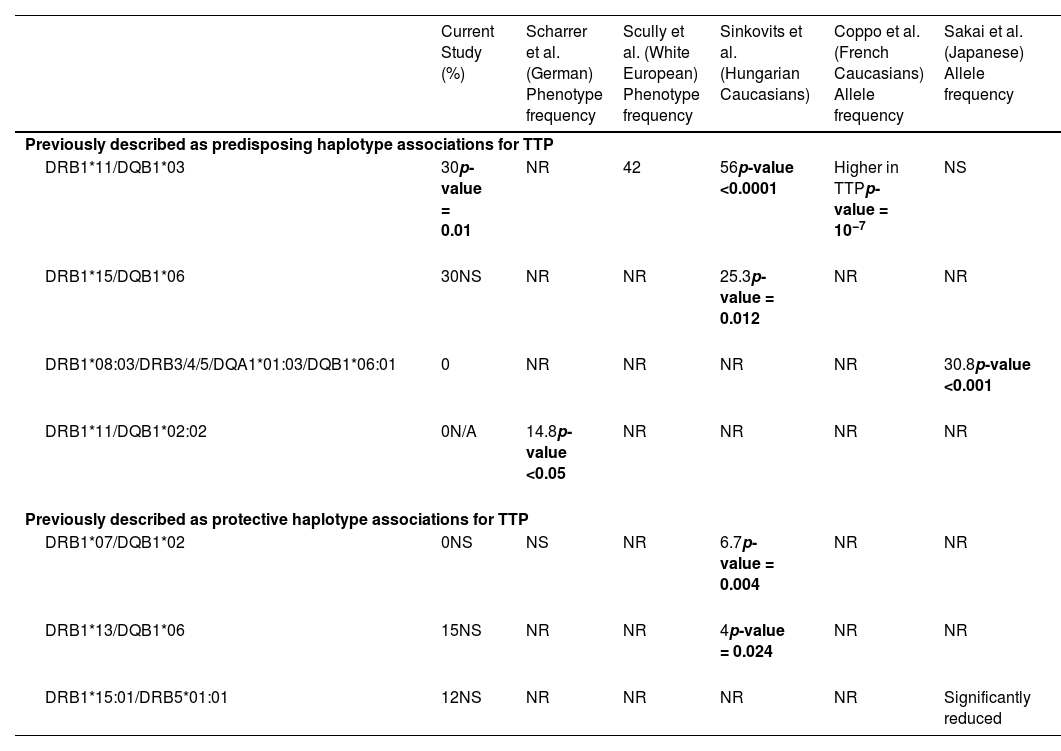
Haplotype frequencies noted in acquired thrombotic thrombocytopenic purpura patients in our study in comparison with the prior reported studies.
TTP: Thrombotic thrombocytopenic purpura.
HLA-DQB1*03:01 commonly associates with HLA-DRB1*11 with the HLA-DRB1*11/DQB1*03:01 haplotype expected to be present in ∼15% of the general population. This haplotype has been reported to be a predisposing haplotype for aTTP.11,12 Similar to prior studies, an increased frequency of this haplotype was observed in the current aTTP patients (30% in aTTP versus 9% in all non-aTTP combined TMA groups; p-value = 0.01). The frequency of this haplotype between aTTP and other individual TMA groups was also compared. Interestingly, this haplotype was not observed in any aHUS patients.
The HLA-DRB1*15/DQB1*06 haplotype was noted in 20–30% of the current cohort, however no statistically significant difference was found for this haplotype among the different TMA groups.
A significant accumulation of HLA-DRB1*11/DQB1*02 was not observed in this study, with only one aTTP patient expressing HLA-DRB1*11/DQB1*02:02. None of the patients in the other TMA groups expressed this haplotype.
Sakai et al.16 reported an increased incidence of aTTP with the presence of HLA-DRB1*08:03/DRB3/4/5*blank/DQA1*01:03/DQB1*06:01. This haplotype was not observed in any of the patients in this cohort, possibly due to racial and ethnic differences.
In addition to the previously described haplotypes, an increased frequency of HLA-DRB1*08/DQB1*03:01/19 was noted in this study in aTTP, particularly for African American patients (21% in aTTP versus 4% in all non-aTTP combined TMA groups; p-value = 0.01). This is a new finding that has not been reported in prior studies.
Protective haplotype associationsNext, the previously described protective haplotype associations were considered (Table 4). Sinkovits et al.12 reported a reduced incidence of HLA-DRB1*07/DQB1*02 in aTTP patients. In the current study, this haplotype was not observed in any of the aTTP patients, while it was noted in 12–15% of non-aTTP (TMA) patients. Although results did not reach statistical significance a trend was noted (p-value = 0.04).
Other previously described protective haplotypes (HLA-DRB1*13/DQB1*06, and DRB1*15:01/DRB5*01:01)12,14 showed no statistically significant differences in the various groups.
The differences between the current study and previous studies maybe due to the racial/ethnic differences in the study populations. As noted in other studies14, African Americans are highly overrepresented in aTTP cohorts in the United States in comparison to European studies, where there is a predominance of Caucasian patients.
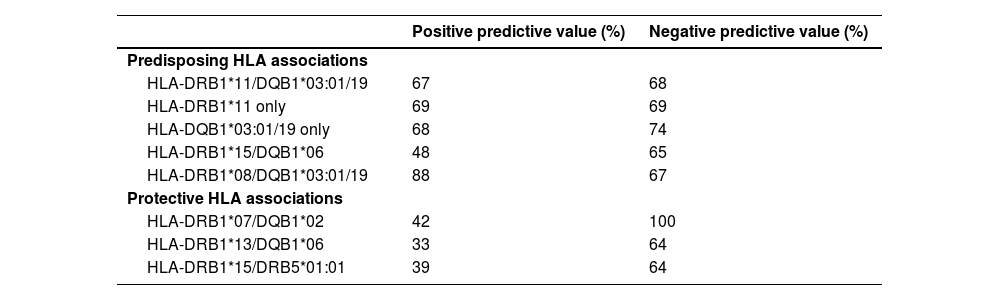
HLA associations with positive and negative predictive valuesTable 6 shows the positive (PPV) and negative predictive values (NPV) of the different HLA associations in aTTP patients versus combined non-aTTP TMA groups.
HLA haplotype associations and Positive and Negative predictive value for acquired thrombotic thrombocytopenic purpura versus Combined non-acquired thrombotic thrombocytopenic purpura thrombotic microangiopathies categories.
The PPV and NPV for the presence of HLA-DRB1*11/DQB1*03:01/19 in aTTP patients were 67% and 68%, respectively. PPV and NPV for presence of HLA-DRB1*11 were 69%. PPV and NPV for presence of HLA-DQB1*03:01/19 were 68% and 74%, respectively. The PPV and NPV for the presence of HLA-DRB1*08 and HLA-DQB1*03:01/19 were 88% and 67%, respectively.
The presence of the protective HLA-DRB1*07/DQB1*02 haplotype provided 100% NPV and 42% PPV for the presence of aTTP.
Other haplotype associations did not provide useful individual PPV or NPV values (see Table 6).
HLA associations and plasmic scoreThe PLASMIC score was calculated for all patients using the previously described criteria.5 PLASMIC scores of 0–4, 5, and 6–7 correspond to low, intermediate, and high probability of aTTP, respectively.
In the current study, a PLASMIC score of 6–7 demonstrated 69% PPV and 95% NPV. The PPV and NPV of HLA associations were overall similar, although slightly better than the PLASMIC score (PPV: 67–88% vs. 69%; NPV: 100% vs. 95%).
In this study, combining the presence of various predisposing alleles, i.e., HLA-DRB1*11, HLA-DQB1*03:01/19, or HLA-DRB1*08 with a PLASMIC score of 6–7 in patients with aTTP allowed for an even higher PPV (90–100%) (Table 7). Thus, combining HLA associations and PLASMIC score 6–7 allowed for higher PPV and NPV than either of them alone.
DiscussionOnly a few prior studies have described HLA associations in aTTP.9-12,16 These studies compared the HLA associations in patients with aTTP to control populations. These prior studies were primarily performed with Caucasian populations with one study being carried out in the Japanese population. In comparison, the current study consists of both Caucasian and non-Caucasian subjects, with an overrepresentation of African American subjects. An overrepresentation of African American subjects has been previously reported by the aTTP registry in the United States.14 A recent study noted that race affects overall relapse risk and response to rituximab in aTTP (Chaturvedi et al.17), further emphasizing the importance of the current study for determining HLA associations in TMA in the United States. In addition, this study included other (non-aTTP) TMA patients in an effort to identify any unique HLA associations that would aid in the differential diagnosis of TMA.
Prior studies have shown an association of HLA-DRB1*11 with autoimmune diseases such as systemic sclerosis, juvenile chronic arthritis, and sarcoidosis.18-20 An increased incidence of HLA-DRB1*11 was also noted in aTTP patients in the European population (Scharrer et al., Scully et al., Sinkovits et al., Coppo et al.),9-12 although Sakai et al.16 did not see this trend in the Japanese population. An increased incidence of HLA-DQB1*03:01 has also been reported in prior studies (Scharrer et al., Scully et al., Sinkovits et al.),9,10,12 although the results did not reach statistical significance in the study by Scharrer et al.9 Scully et al.10 further suggested that the incidence of HLA-DRB1*11 is increased in aTTP due to it being in linkage-disequilibrium with HLA-DQB1*03:01.10,11 Scully et al. reported that 27.6% of aTTP patients were HLA-DQB1*03:01 positive but HLA-DRB1*11-negative, suggesting that HLA-DQB1*03:01 plays the dominant role in disease pathogenicity, while HLA-DRB1*11 is merely present due to its linkage-disequilibrium association with HLA-DQB1*03:01. The results of this study show similar findings with increased HLA-DRB1*11 and DQB1*03:01/19 allele frequencies and HLA-DRB1*11/DQB1*03:01/19 haplotype frequency in aTTP patients as compared with other TMA. Similar to previous studies, a subset of patients had HLA-DQB1*03:01/19 without HLA-DRB1*11 in the current cohort. This was seen in African American patients (7 of 11 patients expressed HLA-DQB1*03:01/19 without HLA-DRB1*11) while all of the Caucasian patients expressed both HLA-DQB1*03:01/19 and HLA-DRB1*11 (Supplemental Table 2). To further elucidate the role of these alleles/haplotypes, the PPV (Table 6) was calculated for the presence of individual alleles [HLA-DRB1*11 (69%), HLA-DQB1*03:01/19 (68%)], as well as the HLA-DRB1*11/DQB1*03:01/19 haplotype (67%) and were found to be similar to that of the PLASMIC score (69%). Combining the presence of HLA-DRB1*11 and HLA-DQB1*03:01/19 with a PLASMIC score of 6–7 in patients with aTTP allowed for a higher PPV (90%). Interestingly, the evaluation of the HLA-DQB1*03:01/19 allele in patients with aTTP and a PLASMIC score of 6–7 provided a PPV of 93%. Perhaps the presence of HLA-DQB1*03:01/19 is the most important HLA association with aTTP, in agreement with Scully et al. Thus, combining PLASMIC score with specific HLA alleles helps in distinguishing aTTP from other TMA.
It was also of note that none of the current patients with aHUS showed the presence of the HLA-DRB1*11/DQB1*03:01/19 haplotype, and thus the absence of this haplotype may be useful in differentiating aHUS from aTTP, where its presence correlates with aTTP.
Sakai et al. observed an increased incidence of HLA-DRB1*08:03 along with HLA-DRB1*08:03/DRB3/4/5*blank/DQA1*01:03/DQB1*06:01 in patients with aTTP.14 This haplotype and the HLA-DRB1*08:03 allele appear to be unique to the Japanese population, since none of the other studies noted this association. In the current cohort, a statistically significant increase in frequency of HLA-DRB1*08 was observed in aTTP patients, but none of these patients had HLA-DRB1*08:03. Six patients had HLA-DRB1*08:04 (one patient was homozygous) and one patient had HLA-DRB1*08:06. An increased incidence of HLA-DRB1*08/DQB1*06 was not observed either. No such trend was noted in other TMA groups. HLA-DRB1*08 commonly associates with HLA-DQB1*03:01/19 particularly in the African American population. In this study, 7 of 33 patients (all African American) with aTTP expressed HLA-DRB1*08/DQB1*03:01/19. Only two of the 53 (also African American) non-aTTP TMA patients expressed HLA-DRB1*08/DQB1*03:01/19. This haplotype association with aTTP has not been described previously, but it is likely that HLA-DQB1*03:01/19 is the primary association and the presence of HLA-DRB1*08 appears to be due to its linkage disequilibrium with HLA-DQB1*03:01/19.
Similar to Scully et al.10 an increased incidence of HLA-DRB3 (DR52) was observed in aTTP patients of the current cohort. Since HLA-DR52 commonly associates with HLA-DRB1*11, the apparent increased incidence of HLA-DR52 observed in this study could be due to its linkage-disequilibrium with HLA-DRB1*11/DQB1*03:01/19 (HLA-DRB1*11 and DQB1*03:01/19 versus DR52: p-value = 0.032; HLA-DQB1*03:01/19 versus DR52: p-value = 0.619).
Similar to Scully et al.10 this study found that HLA-DRB4 (DR53) appears to have a protective association for aTTP due to its low occurrence in the patient population (less than expected in the general population), whereas it is present in non-aTTP patients as expected in the general population.
Sinkovits et al.12 reported a reduced incidence of HLA-DRB1*07/DQB1*02 in aTTP patients. This haplotype was not observed in any aTTP patients in the current study, thus, the presence of this haplotype probably rules out the presence of aTTP (NPV: 100%).
Other statistically significant associations reported by Sinkovits et al.12 i.e. (i) increased frequencies for HLA-DRB1*14, and DRB1*15, and (ii) decreased frequencies for DRB1*13 in aTTP patients, were not observed in the current cohort.
Scharrer et al.9 reported an increased association of HLA-DQB1*02:02 (20% in aTTP versus 1.2% in control group; p-value <0.001); this association was not found in aTTP patients in this cohort. However, an increased presence of HLA-DQB1*02:02 was found in patients with aHUS.
Sakai et al. reported increased frequencies for HLA-DQA1*01:03 and DQB1*06:03, and a decreased frequency for HLA-DRB5*01:01.16 No such associations were reported by other studies or observed in the current study.
In summary, patients with aTTP are more likely to express HLA-DRB1*08, HLA-DRB1*11, HLA-DQB1*03:01/19, and HLA-DRB3, either as individual alleles or the HLA-DRB1*11/DQB1*03:01/19/DRB3 haplotype. They are less likely to express the HLA-DRB1*07/DQB1*02/DRB4 haplotype. Combining HLA associations with PLASMIC score achieved higher PPV (90%) and NPV (100%). Based on these findings, we propose an algorithm (Figure 1) that includes calculating a PLASMIC score and performing rapid HLA typing at the time of initial presentation, while waiting for ADAMTS13 results.
We recommend proceeding with TPE (and/or other alternate therapies i.e., caplacizumab, or recombinant ADAMTS13 therapy when available) in patients with a PLASMIC score of 6 or more. The presence of any predisposing and absence of protective HLA associations, noted on HLA typing further strengthens the likelihood of aTTP (combined PPV: 90%). Likewise, in patients with a PLASMIC score of <6, the absence of predisposing and presence of protective HLA associations further strengthens that aTTP is unlikely (NPV: 100%). Additional testing (complement levels, complement genetic testing etc.) should be performed in these patients if not already requested at initial presentation. In patients with a PLASMIC score of <6, but presence of predisposing and absence of protective HLA associations, it may be reasonable to treat as if it were aTTP until ADAMTS13 results are available. This algorithm allowed us to identify two additional aTTP patients for TPE that would have been missed based on using the PLASMIC score alone (<6). Now that therapeutic ADAMTS13 is available (ADZYMA, ADAMTS13, recombinant-krhn, Takeda®)21 for clinical use in cTTP, it is only a matter of time before it is more generally available and thus it becomes increasingly important not to miss the aTTP cases where PLASMIC scores may not lead to immediate treatment. Likewise, this algorithm allowed us to identify four additional non-aTTP TMA patients that would have undergone treatment for aTTP based on PLASMIC score (6–7) alone, when it may not have been the appropriate treatment.
There are a number of strengths and potential limitations of this study. Strengths of the study include (i) a study population comprising both Caucasian and non-Caucasian subjects consistent with the patient population found in the United States; (ii) inclusion of non-aTTP TMA subjects in the study to determine any unique HLA associations that could aid in the differential diagnosis of TMA; (iii) determining PPV and NPV of the identified associations and comparing them with other indicators (PLASMIC score). Further, the study results are similar to previously published reports. Potential limitations include the small sample size, however, given the overall low incidence of TMA, it is difficult to enroll large number of patients at a single institution. Multi-institution studies are needed to further elucidate the role of HLA associations in TMA.
ConclusionThis study supports the previously identified HLA associations with aTTP, namely increased frequencies of HLA-DRB1*11, HLA-DQB1*03:01/19, HLA-DRB1*08 and HLA-DRB3, and decreased frequencies of HLA-DRB4, HLA-DRB1*07 and HLA-DQB1*02. In addition, combining the presence of HLA-DRB1*11/DQB1*03:01/19 and absence of HLA-DRB1*07/DQB1*02 with the PLASMIC score gives higher PPV and NPV for identifying aTTP, distinguishing it from other types of TMA, and initiating definitive therapy. Other novel findings of this study include (i) increased incidence of HLA-DQB1*02:02 and absence of HLA-DRB1*11/DQB1*03:01/19 in aHUS patients; (ii) presence of HLA-DRB1*07/DQB1*02 does not confer protection against the occurrence of other TMA as it does for aTTP.
CRediT authorship contribution statementSoumya Pandey: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Akul Shrivastava: Formal analysis. Yanping Izak Harville: Formal analysis. Michele Cottler-Fox: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Terry O. Harville: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.