Hematologic abnormalities are frequent among persons living with HIV (PLWH). The bone marrow aspirate (BMA) and biopsy (BMB) are commonly performed in the diagnostic approach of patients with unexplained cytopenias. Changes in antiretrovirals, supportive therapy and increased life expectancy have modified the distribution and etiology of cytopenias, questioning their use. Our aim was to analyze the diagnostic yield of BMA, BMB and marrow cultures for the evaluation of cytopenias in PLWH.
MethodsThis was a retrospective cohort of ≥ 18-year-old PLWH undergoing bone marrow assessment (MA) for the evaluation of cytopenias between January 2002 and December 2015.
ResultsA total of 236 cytopenic events were analyzed, 47.9% being PLWH who had a longstanding diagnosis (≥ 1 year). Adherence to antiretrovirals was 63.5%. Anemia was seen in 91.9% and pancytopenia in 39%. Common presentations included fever (52.1%), weight loss (42.8%) and adenopathies (28.8%). Median days from detection to MA was 5 (0 – 63 days). Most common etiologies were non-HIV infectious diseases (31.4%) and benign/malignant hematologic diseases (26.3%). The diagnostic yield was 16.1% for BMA, 20.3% for BMB, 30.5% for both and 35.6% when cultures were added. Patients most likely to have conclusive MA were those with moderate/severe thrombocytopenia (p = 0.007). Fever, splenomegaly, and low CD4+ counts were associated with infectious etiologies, while hematologic diagnoses were related to the presence of adenopathies.
ConclusionAs a minimally invasive intervention, the MA has a high yield for identifying the etiology of cytopenic events in PLWH, being conclusive in one in three patients. Early performance could lead to prompt diagnosis and timely therapy initiation.
Hematologic abnormalities are frequent among persons living with human immunodeficiency virus (PLWH) and are considered by some authors as being reported before the virus itself.1,2 The most common hematologic finding is the presence of peripheral blood cytopenias. In the antiretroviral therapy (ART) era, anemia has been described as the leading cytopenia reported in approximately 50%, followed by leukopenia and thrombocytopenia.3,4
Cytopenic events, particularly if severe, have been associated with advanced stages of disease, increased disease progression and decreased overall survival in PLWH. These events are pathophysiologically explained by one or more of the following mechanisms: bone marrow hypoproliferation, ineffective hematopoiesis and/or peripheral cell destruction.5
Coinfections, benign and malignant hematologic diseases, autoimmune processes, drug toxicity, micronutrient deficiency and the human immunodeficiency virus (HIV) itself are causes that explain cytopenias.6 Most prevalent specific etiologies in PLWH include disseminated opportunistic infections, mainly Mycobacterium sp. and Histoplasma capsulatum, and Hodgkin and non-Hodgkin lymphomas.7–9
Given the broad differential diagnoses, determining the ideal ancillary testing that can optimize rates of achieving a definitive and accurate etiologic diagnosis is of utmost importance. Traditionally, bone marrow aspirate (BMA) and biopsy (BMB) have been performed within the diagnostic approach of patients with unexplained cytopenias.10 However, its diagnostic performance has not been well defined for PLWH, with reports of the diagnostic yield being widely variable, between 23% and 47%.11–17
It is important to consider that, given the constantly changing scene of HIV, reports across time vary widely according to the temporality and clinical scenario in which these were conducted. Changes in ART and supportive therapy, as well as an increased life expectancy, have modified the number and distribution of cytopenia differential diagnoses, questioning the current use of BMA and BMB.7 The aim of this study was to evaluate the role of bone marrow testing to establish an effective framework and model for the evaluation of cytopenias in PLWH at an HIV referral center in the ART era.
MethodsThis was a retrospective, single-center, cohort study that included all consecutive ≥ 18-year-old PLWH who underwent bone marrow examination as part of the diagnostic evaluation of a cytopenic episode between January 2002 and December 2015. Clinical, microbiological and pathological data were reviewed from the electronic and/or physical medical records. Patients with incomplete records and/or pregnant women were excluded. The study was approved by our center's Institutional Review Board.
Patients were assigned to one of three groups, according to the time from diagnosis of HIV to the cytopenic event: Group A patients, with more than one year, Group B patients, between one month and one year, and Group C, whose diagnosis of HIV was concomitant with that of cytopenia. Cytopenias were defined as: anemia with hemoglobin < 13g/dL for males and < 12g/dL for females, leukopenia with a total leukocyte count < 4×103 cells/μL and thrombocytopenia with a platelet count < 150 × 103 cells/μL. Thrombocytopenia was graded as mild if < 150 × 103 cells/μL, moderate if <100 × 103 cells/μL and severe if < 50 × 103 cells/μL.
Bone marrow aspirate, bone marrow biopsy and bone marrow culture samples were obtained within the same procedure. Routinely, Ziehl–Neelsen (ZN), periodic acid-Schiff (PAS) and Grocott staining were performed in all bone marrow samples. The decision to add bone marrow cultures was made at the treating physician's discretion. The BMA, BMB and bone marrow cultures were defined as conclusive when the final report matched the final diagnosis for the evaluated cytopenia.
Statistical analysisNominal and ordinal variables were described in terms of frequencies and percentages. Quantitative data were described in terms of medians and ranges. Association between categorical variables was evaluated with the Chi-square test for independence. Multivariate analysis for factors associated with a specific etiology was performed using logistic regression analysis. All calculations were performed with SPSS, version 25 (IBM Corp, Armonk, NY).
ResultsThe inclusion criteria were met by 203 patients in whom a total of 236 bone marrow examinations were performed for the diagnostic evaluation of cytopenic episodes. The median age at the time of the initial evaluation was 36 years (18 – 78 years) and 86.7% (n = 176) were male. Regarding the time from the diagnosis of HIV, Group A represented 47.9% (n = 113) of the patients, Group B, 32.2% (n = 76) and Group C, 19.9% (n = 47). Therapeutic adherence to ART was 63.5% (n = 120) in those previously known to be HIV seropositive at the time of the evaluated cytopenic event.
The most common cytopenia was anemia in 91.9% (n = 217) and pancytopenia was observed in 39% (n = 92) of the cases. Median peripheral blood counts were: hemoglobin 9.25 g/dL (2.4 – 16g/dL), total leukocyte count 3.05×103 cells/μL (0.1 – 73.5×103 cells/μL), absolute neutrophil count 1,820/μL (3 – 41,900/μL), absolute lymphocyte count 498/μL (0 – 7,371/μL), and platelets 109×103 cells/μL (2 – 522×103 cells/μL). The observed median CD4+ count was 60 cells/μL (1 – 1,150 cells/μL). Regarding the clinical findings, the patients presented with: fever in 52.1% (n = 123), weight loss in 42.8% (n = 101), adenopathies in 28.8% (n = 68), splenomegaly in 23.7% (n = 56), hepatomegaly in 23.3% (n = 55), diarrhea in 16.5% (n = 39) and skin lesions in 15.7% (n = 37), while 17.4% (n = 41) were asymptomatic. The median number of days from cytopenia detection to BMA/BMB was 5 days (0 – 63 days). Additional demographic and clinical characteristics are presented in Table 1.
Patient baseline demographic and clinical characteristics subgrouped by time from HIV+ diagnosis.
ART: antiretroviral therapy.
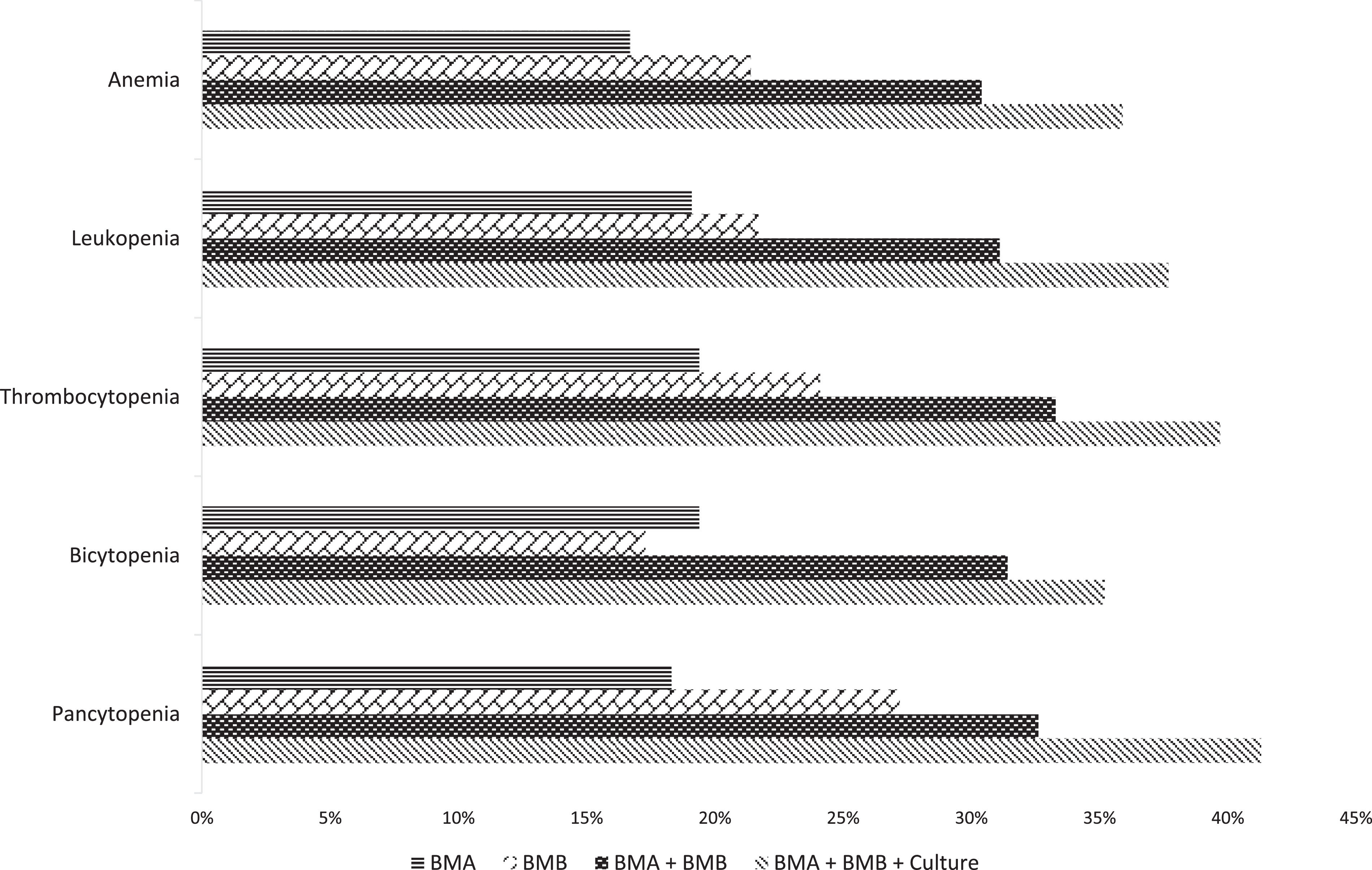
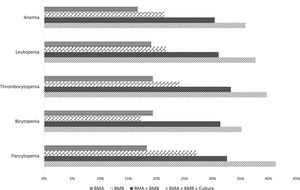
The diagnostic yield was 16.1% (n = 38) for BMA, 20.3% (n = 48) for BMB and 30.5% (n = 72) when associating both procedure results. In 69.5% (n = 164) of the cases, bone marrow cultures were simultaneously obtained at the time of the BMA and BMB, increasing the diagnostic yield to 35.6% (n = 84). The only factor associated with a higher likelihood of a conclusive BMA/BMB was presenting with moderate or severe thrombocytopenia (OR 2.09, 95%CI 1.22 - 3.59; p = 0.007). The associated diagnostic yield for BMA, BMB and both procedures and cultures, according to the type of cytopenia, is shown in Figure 1.
From the group of patients with a non-conclusive BMA/BMB (n = 152), an additional tissue biopsy was obtained in 39.5% (n = 60). The most commonly biopsied tissues were lymph nodes in 40% (n = 24), skin in 10% (n = 6) and spleen in 6.7% (n = 4). Of these, 51 were conclusive and led to a diagnosis in an additional 21.6% of the study population.
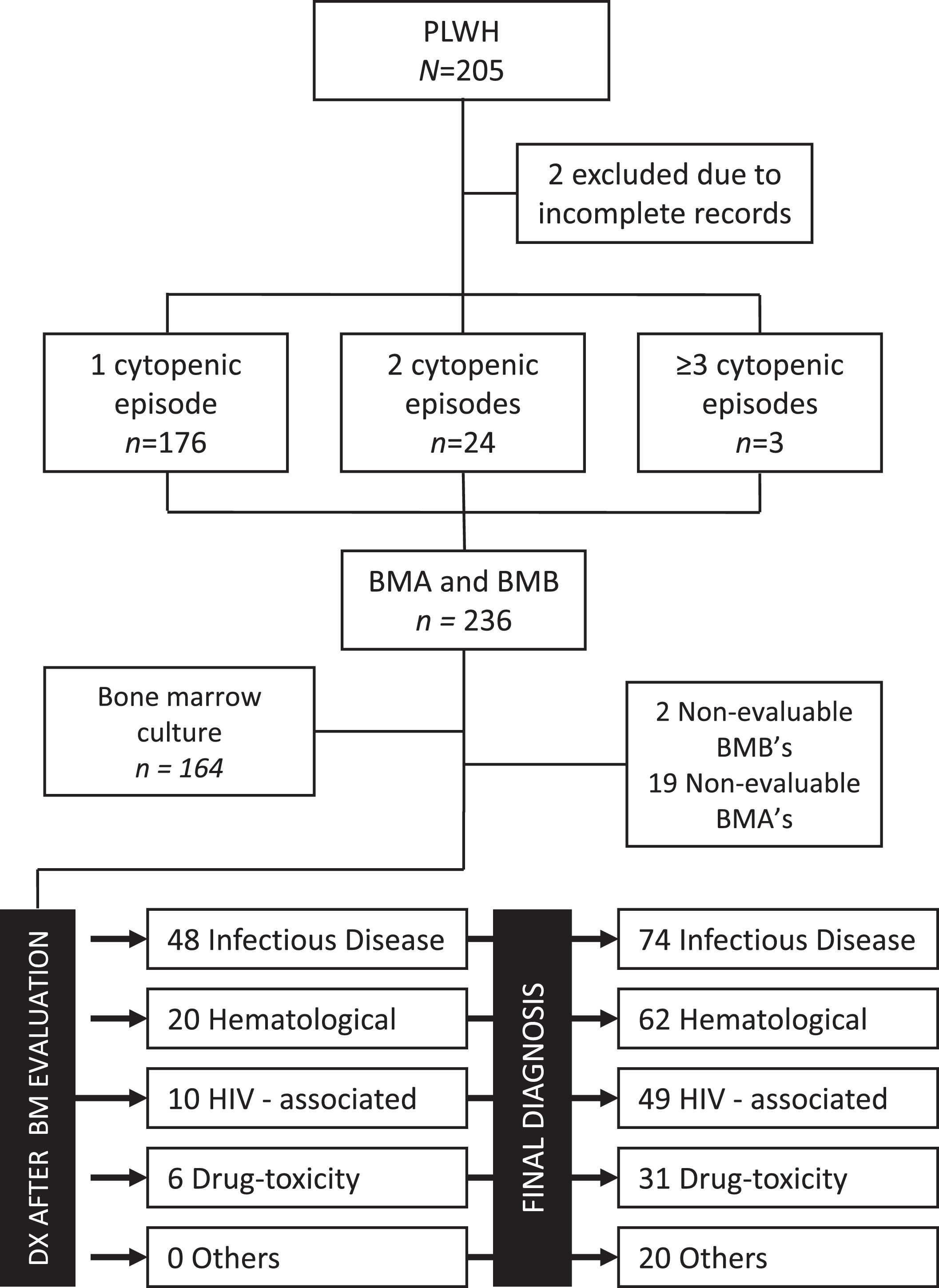
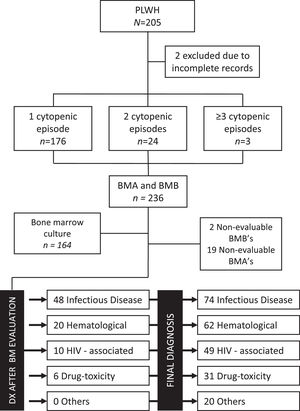
Final diagnoses after the cytopenia evaluation included: non-HIV infectious diseases in 31.4% (n = 74), benign or malignant hematologic diseases in 26.3% (n = 62), HIV-associated cytopenias in 20.8% (n = 49), drug-toxicity in 13.1% (n = 31) and micronutrient deficiency or non-determined in 8.5% (n = 20). The patient diagnostic flow is shown in Figure 2. The two most prevalent infectious diseases were Mycobacterium sp. (n = 28) and Histoplasma capsulatum (n = 27) and the most common hematologic disease was non-Hodgkin lymphoma (n = 33). According to the time from the HIV diagnosis, a trend towards increased infectious etiologies in Groups B and C was seen. On the contrary, a trend towards an increased prevalence of hematologic disorders and drug-toxicities was observed in patients in Group A. Details of disease distribution by subgroups are shown in Table 2.
CONSORT Diagram of patient flow, sample adequacy and final diagnoses for the cohort and for patients with conclusive bone marrow assessment. Abbreviations: BM: bone marrow, BMA: bone marrow aspirate, BMB: bone marrow biopsy, DX: diagnosis, HIV: human immunodeficiency virus, PLWH: persons living with HIV.
Etiology of cytopenic event subgrouped by time from HIV+ diagnosis.
| Diagnosis | Group A n = 113 | Group B n = 76 | Group C n = 47 | p |
|---|---|---|---|---|
| Infectious disease, n (%) | 28 (24.8) | 27 (35.5) | 19 (40.4) | 0.096 |
| Tuberculosis | 7 (25) | 14 (51.9) | 7 (36.8) | |
| Histoplasmosis | 7 (25) | 9 (33.3) | 11 (57.9) | |
| Parvovirus B19 | 3 (10.7) | 1 (3.7) | - | |
| Others* | 11 (39.3) | 3 (11.1) | 1 (5.3) | |
| Hematologic disease, n (%) | 35 (31) | 17 (22.4) | 10 (21.3) | 0.288 |
| HIV-associated, n (%) | 18 (15.9) | 16 (21.1) | 15 (31.9) | 0.076 |
| Drug toxicity, n (%) | 18 (15.9) | 11 (14.5) | 2 (4.3) | 0.126 |
| Other / non-determined, n (%) | 14 (12.4) | 5 (6.6) | 1 (2.1) | 0.081 |
Of the 60 patients that required an additional biopsy, diagnosis was hematologic in 63.3% (n = 38), including 28 non-Hodgkin and 8 Hodgkin lymphomas, HIV-associated in 20% (n = 12) and infectious in 15% (n = 9), while one patient with Kaposi sarcoma had cytopenias determined to be drug toxicity-related.
The multivariate analysis of the correlation between the clinical and laboratory characteristics and the final diagnosis showed that the risk factors associated with infectious disease as the cause of a cytopenic event included fever (p < 0.001), splenomegaly (p = 0.04) and CD4+ cell count < 200 cells/μL (p = 0.02). Likewise, risk factors associated with a hematologic etiology of the evaluated cytopenia included the presence of adenopathies (p = 0.01), the lack of fever (p = 0.01) and the lack of leukopenia (p = 0.001). The only factor associated with an increased likelihood of a drug-related cytopenia was the lack of weight loss (p = 0.003), though trends for the lack of both adenopathies and hepatosplenomegaly were observed. No correlation between risk factors and HIV-associated cytopenias or cytopenias of unknown origin was evidenced. Detailed information on the univariate and multivariate analyses can be found in Table 3.
Univariate and multivariate correlation analysis between clinical and laboratory findings and cytopenia etiology.
CI: confidence interval.
This study represents the largest cohort to date that has undergone the analysis of the role of a comprehensive bone marrow evaluation in the diagnostic approach to cytopenic events in PLWH in a Latin American country. Though multiple groups have reported their experience, most of the available literature has been focused on the assessment of cytopenias in association with febrile episodes, patients with an infectious etiology as the main diagnostic suspicion, and has been restricted to small sample sizes.12,14–17
Our population is comparable to that of prior reports on demographic, clinical and immunologic baseline characteristics. Concerning the time from the diagnosis and therapeutic intervention, our cohort included patients diagnosed with HIV for more than one year in 50% and concomitant diagnosis at time of the cytopenic event in 20%. Despite all patients having been included in the ART era, only 64% of non-newcomers were on active therapy, this being consistent with rates of therapeutic adherence seen in other reports, facilitating extrapolation of our results to the real-world scenario.15–17
The observed diagnostic yield of the complete bone marrow evaluation was consistent with that of the published literature, reaching a conclusive diagnosis for one in every three patients evaluated with this modality. Importantly, despite studies having focused on the diagnostic yield of the BMB rather than that of the BMA, our results demonstrate that the inclusion of the BMA and cultures within the same procedure can improve the test performance. As evidenced in Figure 1, the diagnostic yield increased in a stepwise fashion for all evaluated cytopenias, being particularly efficient for patients presenting with pancytopenia or moderate-to-severe thrombocytopenia.
The most frequent etiology in our cohort was infectious, both opportunistic and non-opportunistic. All cases except for two, one case of histoplasmosis and one of tuberculosis, were observed in patients with a CD4+ count below 200 cells/μl. Of note, no infection-induced cytopenias were seen in the group of patients with ≥ 500 CD4+ cells/μl. In spite of the low rates of ART adherence, significant differences in the CD4+ cell count were observed among the Groups A, B, and C, possibly explaining the decreased prevalence of infections and increased risk of drug-toxicity and malignant hematologic processes in patients with a longstanding diagnosis (Table 2).
The distribution of cytopenias in the cohort was similar to that found in other reports in the ART era, particularly considering the possible effect of zidovudine-containing regimens on blood counts during the initial years of patient accrual.14,15 No correlation between the type of cytopenia and a specific etiology was observed, with the exception of leukopenia. This is most likely explained by the increased risk of infection in leukopenic patients, particularly if CD4+ cell-depleted. On the other hand, though clinical presentation can often be misleading in PLWH, our findings suggest that clinical characteristics, particularly fever, splenomegaly, adenopathies and weight loss, should guide decision-making in the diagnostic algorithm of this population.
Impact of delayed vs. early diagnostic intervention with bone marrow testing has not been thoroughly assessed in the past. In our study, the median time from detection of cytopenias to performance of the procedure was 5 days, ranging from zero to 63 days. Considering bone marrow assessment as a minimally invasive intervention with a low risk-to-benefit ratio, its diagnostic yield is high, prioritizing its prompt performance to identify the etiology and timely initiation of therapy.
Limitations of our study include its retrospective nature and the inability to retrieve information regarding specific currently active and previously used ART. However, detailed ART history has consistently been lacking in other reports.
In conclusion, bone marrow evaluation with BMA, BMB and bone marrow cultures is an accessible and feasible tool that, if appropriately introduced as a first-tier procedure, could potentially lead to improved diagnostic rates in adequate time frames and, optimistically, lead to better clinical outcomes in this high-risk population.
FundingThis work was partially supported by the NIH-funded Caribbean, Central, and South America network for HIV epidemiology (CCASAnet), a member cohort of the International Epidemiologic Databases to Evaluate AIDS (leDEA) (U01AI069923). This award is funded by the following institutes: Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Cancer Institute (NCI), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Mental Health (NIMH), and the Office Of The Director, National Institutes Of Health (OD).
Conflicts of interestThe authors declare no potential conflict of interests.