The hemogram and hemogram-derivative ratios (HDRs) are becoming markers of the severity and mortality of COVID-19. We evaluated the hemograms and serial weekly HDRs [neutrophil-lymphocyte ratio (NLR), monocyte-lymphocyte ratio (MLR), platelet-lymphocyte ratio (PLR), neutrophil-platelet ratio (NPR) and systemic immune-inflammatory index (SII)] in the survivors and non-survivors of COVID-19.
MethodsWe retrospectively reviewed the medical notes and serial hemograms of real-time reverse-transcription polymerase chain reaction (RT-PCR)-confirmed COVID-19 adults hospitalized from April 2020 to March 2021 from the time of diagnosis to the 3rd week of diagnosis.
ResultsOf the 320 adults, 257 (80.3%) were survivors and had a lower mean age than the non-survivors (57.73 vs. 64.65 years, p < 0.001). At diagnosis, the non-survivors had lower hematocrit (p = 0.021), and lymphocyte (p = 0.002) and basophil (p = 0.049) counts and the hematocrit showed a p-value (Is this what you meant???) of 0.021); higher NLR (p < 0.001), PLR (p = 0.047), NPR (p = 0.022) and SII (p = 0.022). Using general linear models, the survivors and non-survivors showed significant variations with weekly lymphocyte count (p < 0.001), neutrophil count (p = 0.005), NLR (p = 0.009), MLR (p = 0.010) and PLR (p = 0.035). All HDRs remained higher in the non-survivors in the 2nd week and 3rd week of diagnosis and the HDRs were higher in the intubated patients than in the non-intubated patients. The NLR and SII were more efficient predictors of mortality in COVID-19 patients.
ConclusionsThis study shows that serial lymphocyte and neutrophil counts, NLR, PLR, MLR, NPR and SII could serve as good and easily accessible markers of severity and predictors of outcomes in COVID-19 patients and should be used for the monitoring of treatment response.
The novel Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) was first diagnosed in December 2019 and has become a pandemic since March 2020.1 Brazil became the South American epicenter for coronavirus disease (COVID-19) soon after its first case was diagnosed in February 2020, with the highest infection rate occurring in the state of Sao Paulo. To date, there are almost 500 million confirmed cases of COVID-19 globally, 30,152,402 in Brazil and 5,310,464 in the state of Sao Paulo.2,3 The COVID-19 is characterized by marked thrombo-inflammation mechanisms and the cytokine storm has been described as a major marker of prognosis in patients with COVID-19, along with other markers of inflammation and co-morbidities.4,5 Not many centers that attend to these patients have access to sophisticated methods utilized in determining the levels of the cytokines and other markers of systemic inflammation and, in most cases, the methods for determining these cytokines are time-consuming and expensive.
In emergency settings, during hospitalizations and in the outpatient setting, a hemogram is one of the most affordable and accessible minimally invasive investigations that can be very useful in the management of diseases.6,7 Hemogram-derived ratios (HDR), including neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), monocyte-lymphocyte ratio (MLR), neutrophil-platelet ratio (NPR) and systemic immune-inflammation index (SII), may serve as surrogate markers for systemic inflammation and various studies have shown that they could be very useful and reliable inflammatory markers and predictors of the severity and mortality of COVID-19.8-12 Consistently, studies have shown interesting differences in the lymphocyte count and HDRs among the varied clinical spectrum of COVID-19 at diagnosis.9,13 Moreover, lymphopenia, high HDRs and neutrophilia at diagnosis are associated with COVID-19 severity, including the need for intubation and mortality.9,10,12-14 As the hemogram is repeated several times during hospitalizations, we therefore hypothesized that lymphopenia, neutrophilia and these HDRs would significantly worsen in non-survivors of COVID-19 during the consecutive weeks of hospitalization following COVID-19 diagnosis and the monitoring of these markers could help in the management of COVID-19 patients and improve disease outcome.
We analyzed the hemograms and hemogram derivatives (NLR, PLR, MLR, NPR and SII) as surrogate markers of inflammation in survivors and non-survivors of COVID-19 at diagnosis and the following two (2) consecutive weeks of hospitalization at a reference hospital in Campinas.
MethodsIn this retrospective study, we carefully reviewed the electronic medical notes of 320 RT-PCR-confirmed COVID-19 positive adults (18 years and older) hospitalized at the Clinical Hospital of UNICAMP, from April 2020 to March 2021. The Clinical Hospital of UNICAMP is one of the referral centers for COVID-19 patients in the state of Sao Paulo, Brazil. The SARS-CoV-2 infection was confirmed by RT-PCR tests performed with throat and/or nasal swabs or tracheal aspirates at the point of hospitalization or as surveillance screening for patients who were hospitalized for other disease conditions.
The biodata of these adult patients, including the age, gender and duration of the hospitalization, were collected. A minimum of one and a maximum of three hemograms were retrieved for each patient, depending on the duration of the hospitalization as follows: at diagnosis/1st week (W0) of COVID-19 diagnosis, 2nd week (W1) and 3rd week (W2). The routine hemograms of all patients were analyzed using the automated counter XN-9000 (Sysmex Corporation, Kobe, Japan) from the time of the COVID-19 diagnosis. Subsequently, five (5) HDRs were calculated: NLR = absolute neutrophil count (NC)/absolute lymphocyte count (LC); PLR = platelet counts (PC)/LC; MLR = absolute monocyte count (MC)/LC; NPR = NC/PC, and; SII = NC x PC/LC. While all other HDRs do not have units of measurement, the SII is measured in cells/µL.
The main outcomes of the study were recovery from the disease and discharge from the hospital and in-hospital mortality for those who died during hospitalization. There were 257 (80.3%) survivors, i.e., those who recovered from the COVID-19 and were discharged from the hospital and 63 (19.7%) non-survivors, i.e., those who died during hospitalization. The 320 patients were also divided into those who required intubation or not during the hospitalization for COVID-19.
Statistical analyses were performed using the SPSS-22 (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY) and GraphPad Prism-6 (GraphPad Prism Version 6.04 for Windows, GraphPad Software, La Jolla, CA). Unpaired data of Survivors and Non-survivors with COVID-19 patients were compared using Mann-Whitney tests. Categorical variables were compared using χ2 or Fisher´s exact test and the strength of the association was determined using Odds ratios. Associations between continuous variables were determined using the Spearman´s rho correlation analysis. We used general linear models for repeated measures and corrected for the patients who did not stay long enough to have a complete series of blood counts performed. Serial measures were compared within and between groups using univariate and multivariate tests in the general linear models. Univariate binary logistic regression and Area under the Receiver Operating Characteristics (AUC/ROC) curve analyses were used to predict the mortality of COVID-19. All results were considered significant if p < 0.05.
Ethical statementIt was a retrospective cohort study of RT-PCR-confirmed COVID-19 positive patients approved by the Research Ethics Committee of UNICAMP (CAAE # 46644621.0.0000.5404) in accordance with the Declaration of Helsinki of 1975 (revised in 2013) and all participants gave written consent.
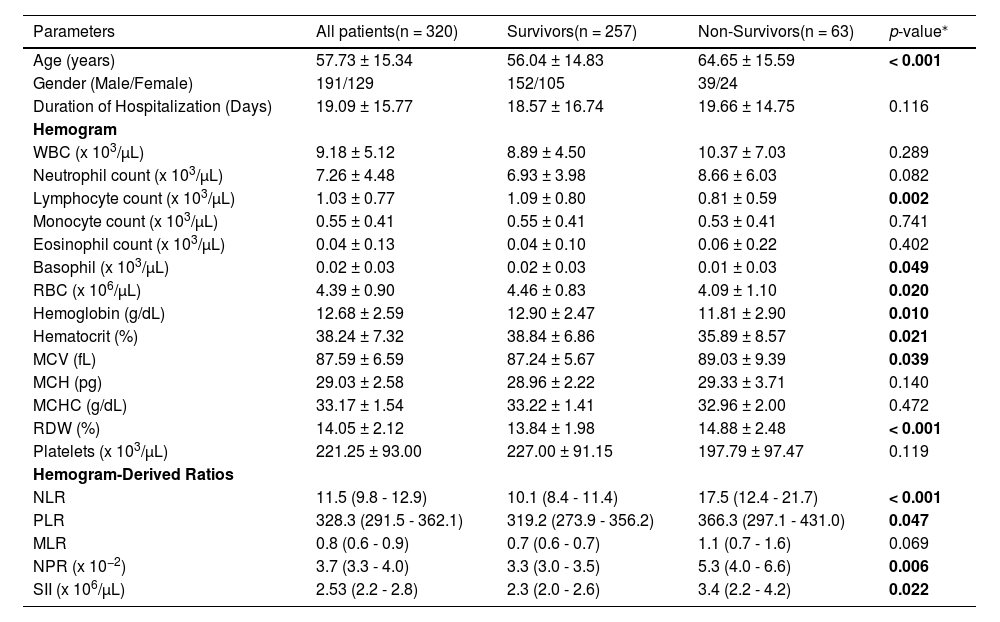
ResultsCharacteristics, hemograms and hemogram-derived ratios of COVID-19 patients at diagnosisA total of 320 patients with a mean age of 57.7 years and a mean duration of hospitalization of 19.1 days post-COVID-19 diagnosis had RT-PCR-confirmed COVID-19. The survivors were 257 (80.3%) and had a lower mean age than the non-survivors [n = 63 (19.7%)] (56.04 vs. 64.65 years, p < 0.001). At diagnosis, the non-survivors had a lower lymphocyte count (p = 0.002), basophil count (p = 0.049) and hematocrit (p = 0.021) and a higher MCV (p = 0.039) and RDW (p < 0.001) than the survivors, Table 1.
Characteristics and hemograms of patients at diagnosis of COVID-19.
| Parameters | All patients(n = 320) | Survivors(n = 257) | Non-Survivors(n = 63) | p-value⁎ |
|---|---|---|---|---|
| Age (years) | 57.73 ± 15.34 | 56.04 ± 14.83 | 64.65 ± 15.59 | < 0.001 |
| Gender (Male/Female) | 191/129 | 152/105 | 39/24 | |
| Duration of Hospitalization (Days) | 19.09 ± 15.77 | 18.57 ± 16.74 | 19.66 ± 14.75 | 0.116 |
| Hemogram | ||||
| WBC (x 103/µL) | 9.18 ± 5.12 | 8.89 ± 4.50 | 10.37 ± 7.03 | 0.289 |
| Neutrophil count (x 103/µL) | 7.26 ± 4.48 | 6.93 ± 3.98 | 8.66 ± 6.03 | 0.082 |
| Lymphocyte count (x 103/µL) | 1.03 ± 0.77 | 1.09 ± 0.80 | 0.81 ± 0.59 | 0.002 |
| Monocyte count (x 103/µL) | 0.55 ± 0.41 | 0.55 ± 0.41 | 0.53 ± 0.41 | 0.741 |
| Eosinophil count (x 103/µL) | 0.04 ± 0.13 | 0.04 ± 0.10 | 0.06 ± 0.22 | 0.402 |
| Basophil (x 103/µL) | 0.02 ± 0.03 | 0.02 ± 0.03 | 0.01 ± 0.03 | 0.049 |
| RBC (x 106/µL) | 4.39 ± 0.90 | 4.46 ± 0.83 | 4.09 ± 1.10 | 0.020 |
| Hemoglobin (g/dL) | 12.68 ± 2.59 | 12.90 ± 2.47 | 11.81 ± 2.90 | 0.010 |
| Hematocrit (%) | 38.24 ± 7.32 | 38.84 ± 6.86 | 35.89 ± 8.57 | 0.021 |
| MCV (fL) | 87.59 ± 6.59 | 87.24 ± 5.67 | 89.03 ± 9.39 | 0.039 |
| MCH (pg) | 29.03 ± 2.58 | 28.96 ± 2.22 | 29.33 ± 3.71 | 0.140 |
| MCHC (g/dL) | 33.17 ± 1.54 | 33.22 ± 1.41 | 32.96 ± 2.00 | 0.472 |
| RDW (%) | 14.05 ± 2.12 | 13.84 ± 1.98 | 14.88 ± 2.48 | < 0.001 |
| Platelets (x 103/µL) | 221.25 ± 93.00 | 227.00 ± 91.15 | 197.79 ± 97.47 | 0.119 |
| Hemogram-Derived Ratios | ||||
| NLR | 11.5 (9.8 - 12.9) | 10.1 (8.4 - 11.4) | 17.5 (12.4 - 21.7) | < 0.001 |
| PLR | 328.3 (291.5 - 362.1) | 319.2 (273.9 - 356.2) | 366.3 (297.1 - 431.0) | 0.047 |
| MLR | 0.8 (0.6 - 0.9) | 0.7 (0.6 - 0.7) | 1.1 (0.7 - 1.6) | 0.069 |
| NPR (x 10−2) | 3.7 (3.3 - 4.0) | 3.3 (3.0 - 3.5) | 5.3 (4.0 - 6.6) | 0.006 |
| SII (x 106/µL) | 2.53 (2.2 - 2.8) | 2.3 (2.0 - 2.6) | 3.4 (2.2 - 4.2) | 0.022 |
NLR neutrophil-lymphocyte ratio, PLR platelet-lymphocyte ratio, MLR monocyte-lymphocyte ratio, NPR neutrophil-platelet ratio, SII systemic immune-inflammation index.
The non-survivors had a higher NLR [17.5 (95%CI = 12.4 - 21.7) vs. 10.1 (95% = 8.4 - 11.4), p < 0.001] and a higher PLR [366.3 (95%CI = 297.1 - 431.0) vs. 319.2 (95%CI = 273.9 - 356.2), p = 0.047] than the survivors. Similarly, the non-survivors had a higher NPR [0.053 (95% = 0.04 - 0.066) vs. 0.033 (95% = 0.03 - 0.035), p = 0.006) and a higher SII [3.4 (95% = 2.2 - 4.2 × 106) vs. 2.3 (2.0 - 2.6 × 106/μL, p = 0.022) than the survivors. However, the MLR was not statistically different between the non-survivors and survivors [1.05 (95%CI = 0.7 - 1.6) vs. 0.68 (95%CI = 0.6 - 0.7), p = 0.069], Table 1.
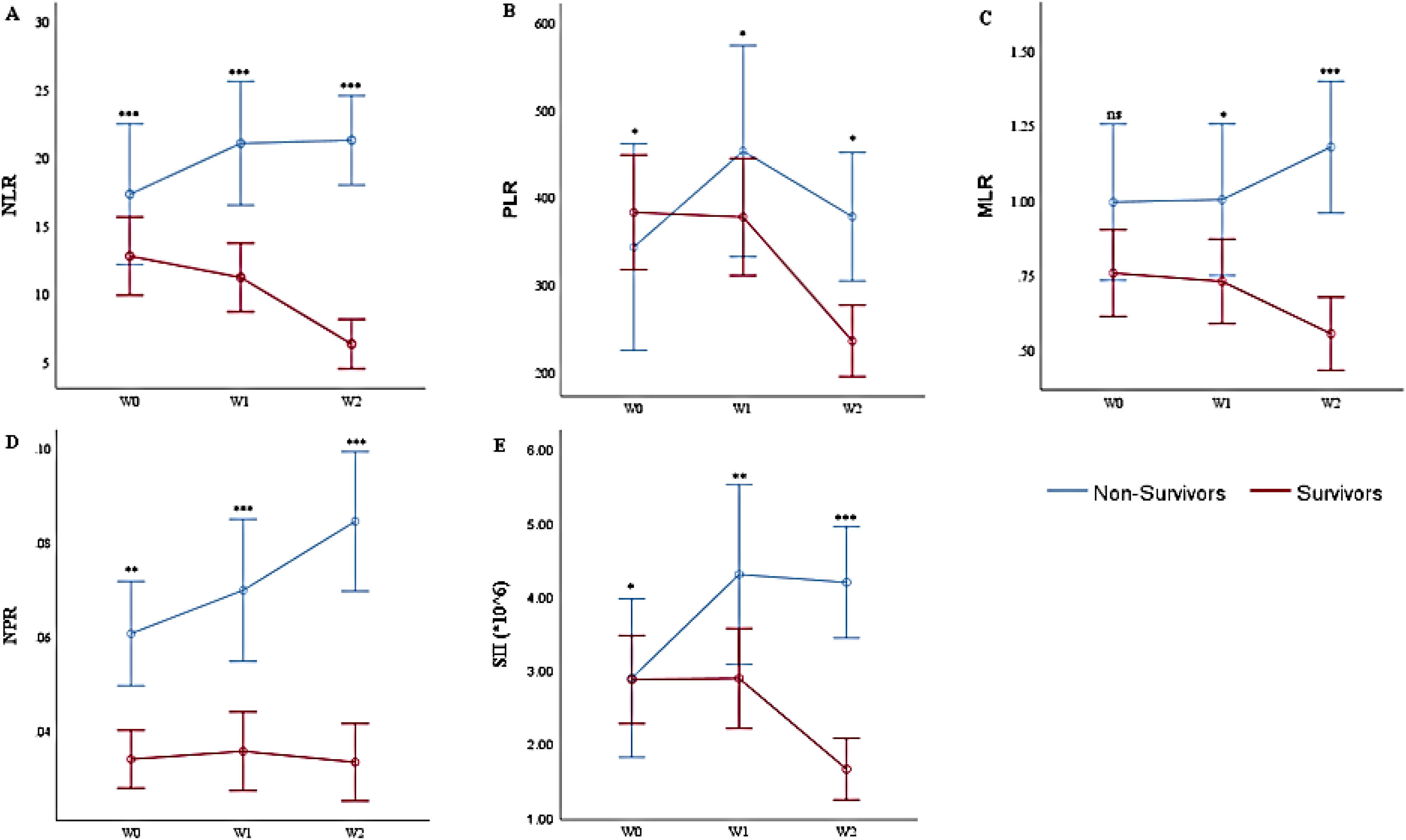
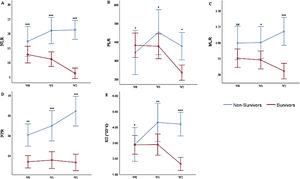
Multivariate analysis of the weekly hemogram-derived ratios of COVID-19 patientsUsing general linear models, we analyzed the consecutive/weekly HDRs of the patients, Figure 1A - E. The model corrected for (by excluding) all patients with less than the complete series of (three) weekly hemograms and HDRs. Thus, there were 174 COVID-19 patients with a complete series of hemograms and HDRs (134 survivors and 40 non-survivors). Multivariate tests between the survivor and non-survivor groups showed significant variations with serial weekly NLR (p = 0.009), PLR (p = 0.035), MLR (p = 0.010), NPR (p = 0.017) and SII (p = 0.006).
Multivariate Analysis of the Hemogram-Derived Ratios between COVID-19 Survivors and Non-Survivors: A. Neutrophil-Lymphocyte Ratio (NLR); B. Platelet-Lymphocyte Ratio (PLR); C. Monocyte-Lymphocyte Ratio; D. Neutrophil-Platelet Ratio (NPR); E. Systemic Immune Inflammation Index (SII) at diagnosis (W0), 2nd week (W1), and 3rd week (W2) post-diagnosis, ns not significant, * p < 0.05, ** p < 0.01, ***p < 0.001 (Mann-Whitney test). n = 174 (134 survivors and 40 non-survivors).
At diagnosis/1st week of COVID-19 diagnosis (W0), following the correction for those patients without complete series of hemograms/HDRs, the results slightly differ from those earlier presented for all (320) patients at diagnosis. The NLR [17.49 (95%CI = 11.2 - 23.5) vs. 12.92 (95%CI = 10.1 - 15.5)] and NPR [0.06 (95%CI = 0.04 - 0.08) vs 0.03 (95%CI = 0.03 - 0.04)] were higher, while the PLR [342.01 (95%CI = 259.2 - 427.8) vs. 388.41 (95%CI = 312.6 - 454.8)] and SII [2.88 (95%CI = 1.7 - 4.1 × 106) vs. 2.91 (95%CI = 2.3 - 3.5 × 106/μL)] were lower, in the non-survivors (p < 0.001, p = 0.047, p = 0.006 and p = 0.022, respectively). Contrarily, the MLR [1.0 (95%CI = 0.7 - 1.3) vs. 0.77 (95%CI = 0.6 - 0.9)] was not significantly different at diagnosis (p = 0.09).
In the 2nd week (W1) of the COVID-19 diagnosis, the NLR [21.32 (95%CI = 15.1 - 27.1) vs. 11.01 (95%CI = 9.0 - 13.5)], PLR [455.01 (95%CI = 323.6 - 582.9) vs. 375.05 (95%CI = 311.7 - 444.0)], MLR [0.99 (95%CI = 0.7 - 1.3) vs. 0.75 (95%CI = 0.6 - 0.9)], NPR [0.07 (95%CI = 0.05-0.09) vs. 0.04 (95%CI = 0.03 - 0.04)] and SII [4.23 (95%CI = 2.7 - 5.9 × 106) vs. 2.85 (95%CI = 2.3 - 3.5 × 106/μL)] were higher (p < 0.001, p = 0.043, p = 0.022, p = 0.000 and p = 0.001, respectively) in the non-survivors than in the survivors.
In the 3rd week (W2), the NLR [21.56 (95%CI = 15.2 - 27.4) vs. 6.31 (95%CI = 5.4 - 7.4)], PLR [378.78 (95%CI = 264.4 - 492.0) vs. 236.98 (95%CI = 204.2 - 268.1)], MLR [1.19 (95%CI = 0.8 - 1.6) vs. 0.54 (95%CI = 0.5 - 0.6)], NPR [0.08 (95%CI = 0.06 - 0.11) vs. 0.03 (95%CI = 0.03 - 0.04)] and SII [4.12 (95%CI = 2.9 - 5.5 × 106) vs. 1.67 (95%CI = 1.4 - 1.9 × 106/μL)] were higher (p < 0.001, p = 0.043, p < 0.001, p = 0.000 and p = 0.000, respectively) in the non-survivors than in the survivors.
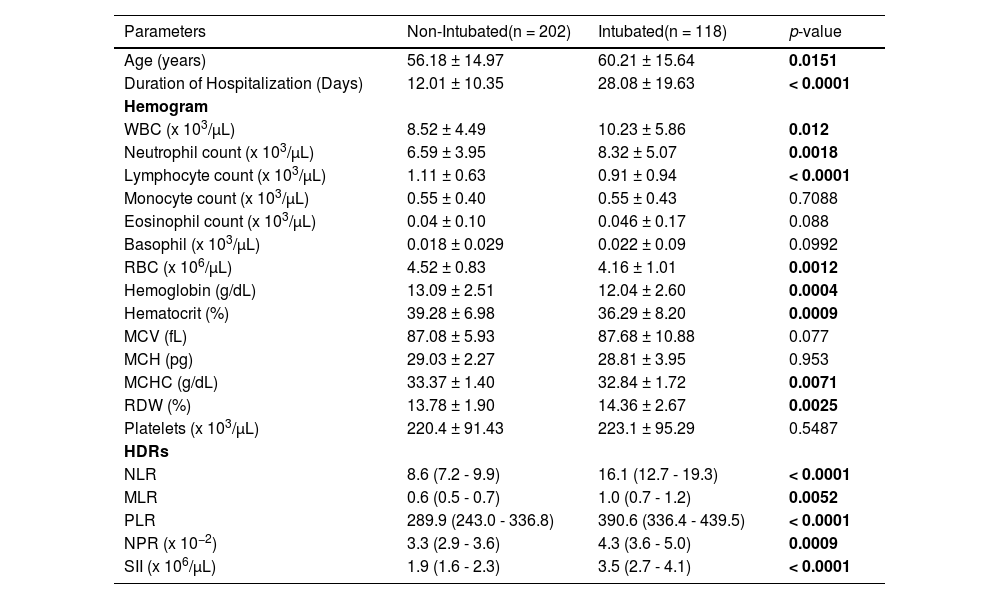
Hemograms and hemogram-derived ratios of intubated and non-intubated COVID-19 patients at diagnosisOf the 320 patients, 118 (36.9%) were intubated during hospitalization, while 202 (63.1%) were not. Of the 118 intubated patients, 60 (50.8%) were survivors and 58 (49.2%) were non-survivors, while out of the 202 non-intubated COVID-19 patients, 197 (97.5%) were survivors and five (2.5%) were non-survivors. Intubation was significantly associated with mortality (χ2 = 103.7, df = 1, p < 0.0001, OR = 38.73, 95%CI = 14.85 - 101.1).
Table 2 summarizes the characteristics and hemogram/HDRs of intubated and non-intubated COVID-19 patients at diagnosis. The intubated patients were older than the non-intubated patients (60.2 vs. 56.2 years, p = 0.0151) and their hospital stay was longer than that of the non-intubated patients (28.1 vs. 12.0 days, p < 0.0001). The leukocyte (p = 0.012) and neutrophil (p = 0.0018) counts were higher in the intubated patients, while the lymphocytes (p < 0.0001) and red cells (p = 0.0012), hemoglobin (p = 0.0004) and hematocrit (p = 0.0009) were lower in the intubated patients.
Hemograms and hemogram-derived ratios of intubated and non-intubated COVID-19 patients at diagnosis.
RBC Red Blood Cell count, MCV Mean Cell Volume, MCH Mean Cell hemoglobin, MCHC Mean Cell Hemoglobin Concentration, RDW Red Cell Distribution Width, WBC White Blood Cell count NLR neutrophil-lymphocyte ratio, PLR platelet-lymphocyte ratio, MLR monocyte-lymphocyte ratio, NPR neutrophil-platelet ratio, SII systemic immune inflammation index. *Mann-Whitney test to compare means between survival and non-survivors. HDRs [mean (95%CI)].
The HDRs (NLR, MLR, PLR, NPR and SII) were all significantly higher in the intubated than in the non-intubated COVID-19 patients at diagnosis (p < 0.0001, p = 0.0052, p < 0.0001, p = 0.0009 and p < 0.0001, respectively), Table 2.
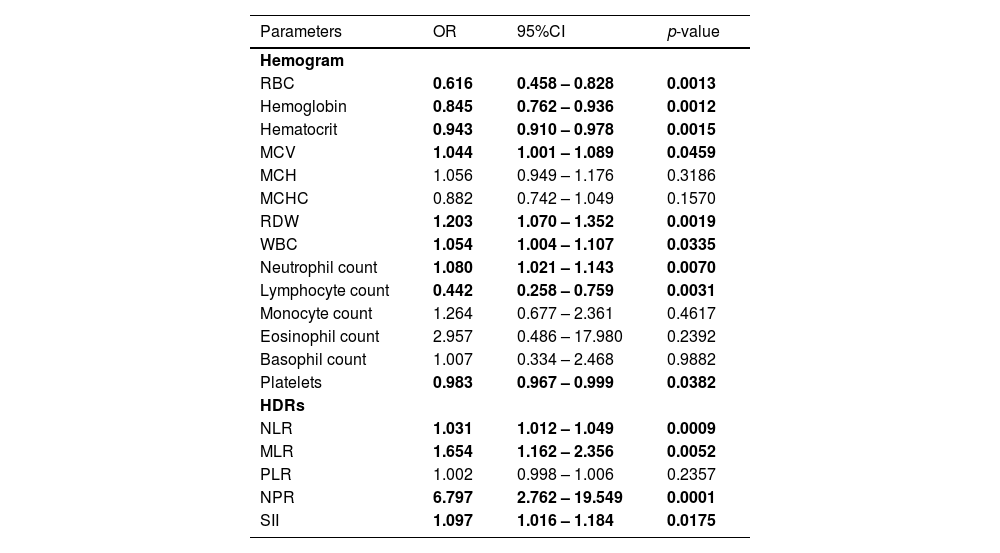
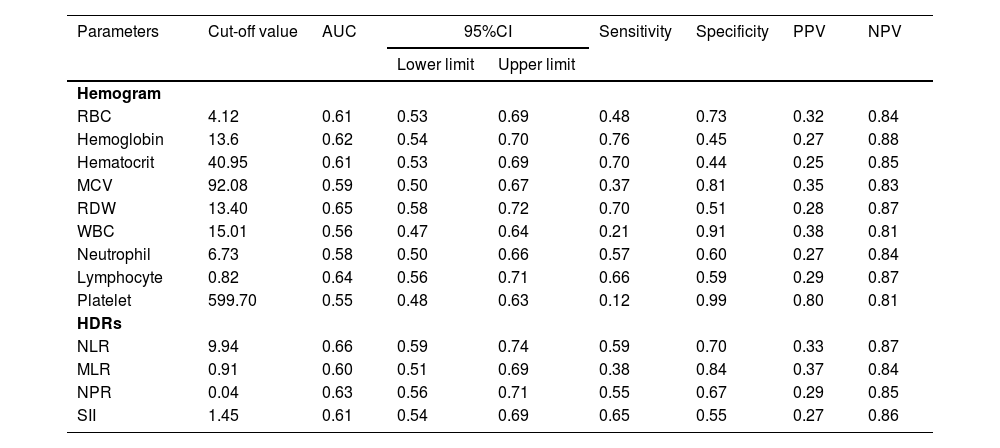
Hemogram and hemogram-derived ratios at diagnosis as predictors of mortality and their diagnostic valuesUsing univariate binary logistic regression analyses and ROC, the hemograms and the HDRs at diagnosis associated with mortality were identified (Tables 3 and 4). The WBC and RBC counts, hemoglobin, hematocrit, MCV and RDW at diagnosis were associated with mortality in these patients, Table 3. Among the HDRs, the NLR, MLR, NPR and SII at diagnosis predicted mortality in the COVID-19 patients. Analyzing the ROC curves for each of these associated factors, the NLR had the highest AUC, while the SII had the highest sensitivity in predicting the mortality in COVID-19 (Table 4).
Hemogram and hemogram-derived ratios at diagnosis associated with mortality in COVID-19 (n = 320).
RBC Red Blood Cell count, MCV Mean Cell Volume, MCH Mean Cell hemoglobin, MCHC Mean Cell Hemoglobin Concentration, RDW Red Cell Distribution Width, WBC White Blood Cell count, NLR neutrophil-lymphocyte ratio, PLR platelet-lymphocyte ratio, MLR monocyte-lymphocyte ratio, NPR neutrophil-platelet ratio, SII systemic immune inflammation index, CI Confidence Interval.
Receiver Operating Characteristics (ROC) curve analysis of hemograms and HDRs at diagnosis to predict mortality in COVID-19 patients (n = 320).
RBC Red Blood Cell count, MCV Mean Cell Volume, RDW Red Cell Distribution Width, WBC White Blood Cell count, NLR neutrophil-lymphocyte ratio, MLR monocyte-lymphocyte ratio, NPR neutrophil-platelet ratio, SII systemic immune inflammation index. AUC Area under the ROC curve, CI Confidence Interval, PPV Positive Predictive Value, NPV Negative Predictive Value.
This study retrospectively analyzed the easily affordable and accessible hemograms and hemogram derivatives (NLR, PLR, MLR, NPR and SII) in survivors and non-survivors of COVID-19 at diagnosis and the following two consecutive weeks of hospitalization after diagnosis at a reference hospital in Campinas. Specifically, the non-survivors had a higher mean age, lower lymphocyte count and hemoglobin and higher neutrophil count, NLR, PLR, NPR and SII than the survivors at COVID-19 diagnosis. During the consecutive weeks, the survivors recovered from lymphopenia and neutrophilia while the non-survivors did not, and the NLR, PLR, MLR, NPR and SII remained higher in the non-survivors. Endotracheal intubation was associated with mortality (p < 0.0001) and the HDRs were higher in the intubated patients than in the non-intubated patients. The HDRs, especially the NLR and SII, were more efficient predictors of mortality in COVID-19 patients.
The data from this study are consistent with previous studies that showed that patients with severe COVID-19 have lymphopenia and that those with higher NLR have poorer outcomes.10,12-16 Lymphopenia appears to be consistent in COVID-19, but the cause is still unclear. Acute recruitment of lymphocytes to the site of infection (mainly the lung) and enhanced sequestration, associated with increased cellular adhesion molecule-1 levels, may contribute to the lymphopenia seen in patients with COVID-19.17 The role of lymphocytes in the pathogenesis and recovery from COVID-19 is buttressed with the persistence of lymphopenia in the non-survivors during the consecutive weeks of hospitalization and the improvement of the lymphocyte count in the survivors during the 2nd and 3rd weeks of COVID-19 diagnosis. These results are consistent with previous studies, which suggest that recovery from lymphopenia is an important determinant of patient recovery from COVID-19.13
Whereas the NLRs and the neutrophil counts declined in the survivors, they were persistently elevated in the non-survivors of COVID-19. Neutrophilia, as well as lymphopenia, are innate immune physiological responses to systemic inflammation that result from cellular redistribution from the marginal pool and apoptosis, respectively, due to the presence of the SARS-CoV-2-related factors.8 A large quantity of reactive oxygen species released from neutrophils induces nuclear damage18 and liberates virus from cells, leading to antibody-dependent cell-mediated viral destruction and cell-specific and humoral immune responses, leading to a marked inflammatory response, which is never resolved due to the persistence of the deranged neutrophil and lymphocyte counts that may have contributed to the mortality of the non-survivors.
Similar to previous reports that show that high PLR levels during hospital admission were associated with severe COVID-19,19 our study shows that the non-survivors had a higher NLR, PLR, NPR and SII than the survivors at the COVID-19 diagnosis. The index study emphasizes that continuous monitoring of the HDRs and other hemogram derivatives could be useful markers of severity and mortality in COVID-19 patients. This is demonstrated by significant improvement in the platelet count and decreasing PLR in the survivors during the consecutive weeks following COVID-19 diagnosis and the subsequent recovery/discharge from the hospital. Whereas the difference in monocyte counts between the survivors and non-survivors was not consistent, the difference in MLR, similar to those of the NLR and PLR, was persistently higher in the non-survivors during the 2nd and 3rd weeks following diagnosis. These findings suggest that the HDRs appear to be consistent and could be more reliable than the absolute cell counts alone in the monitoring of patients during hospitalization for COVID-19. To further buttress the importance of serial monitoring of these HDRs, we found that following general linear models and corrections for those patients without three serial hemograms and HDRs, the SII, a marker of immune/inflammatory response, declined in the survivors in the following two weeks and increased in the non-survivors, suggesting worsening systemic inflammatory response in the non-survivors. Therefore, initiating interventions/treatment that will reduce the inflammatory response would benefit the COVID-19 patients and the serial HDRs would help in monitoring response to such treatments. The role of platelets and monocytes in the pathogenesis of this disease has been suggested by studies that showed that the SARS-CoV-2 infects monocytes and endothelial cells, leading to the cytokine storm, thrombosis and other complex downstream effects.20 This suggests that the platelets and monocytes are being consumed by the persistent SARS-CoV-2 infection and may account for the lower platelet counts in the non-survivors, though this was not statistically significant. This also supports the rationale of the use of anti-thrombotic/anti-coagulation agents in the management of selected high-risk COVID-19 patients, especially during hospitalization.21
A previous study8 observed that older age, lower hematocrit and hemoglobin could have contributed to the mortality in non-survivors. Lymphopenia, and by inference, the immune response is worse in the elderly.22 This is corroborated by the findings in this study, which showed that the LC decreases with age (Supporting Information Table S1) and, as we also demonstrated previously, that the non-survivors had a higher probability of presenting with lymphopenia.23 As in normal aging, there is a progressive declining function in the immune system with age. This immune response decline, in addition to increased co-morbidities (such as hypertension, diabetes mellitus, cancers, etc.), makes the older age group more vulnerable to novel infections, such as the SARS-CoV-2 infection, and leads to the increased age of the non-survivors. The compromised immune response is linked to a decrease in peripheral naïve T and B lymphocytes due to reduced lymphopoiesis24 and loss of expression of the co-stimulatory surface protein CD28, which is a major characteristic of senescent T cells.25 The senescent cells lacking the CD28 then become less susceptible to apoptosis and may worsen cytokine storms in the older age group by secreting more cytokines.25 Furthermore, the lower hemoglobin level in non-survivors means reduced oxygen-carrying capacity of the blood and worsened tissue hypoxemia, the release of reactive oxygen species and end-organ damage, thus leading to poor outcomes.
Overall, four of the hemogram-derived ratios analyzed (NLR, MLR, NPR and SII) from routine hemograms at COVID-19 diagnosis could help predict mortality. All these parameters were significantly higher in the non-survivors than in the survivors at the 3rd week (W2) of COVID-19 diagnosis. This further points to the importance of the ratios as useful predictors of recovery from COVID-19. These ratios were also higher in the intubated patients, regardless of the outcome of the patients. Among all these, as Lopez-Escobar et al.10 reported among Spanish patients, the NLR, having the highest AUC, could be used as an independent predictor of the outcome in COVID-19. Additionally, from the current study, the SII at diagnosis having the highest sensitivity and lowest specificity, with a similar AUC as the NLR, could be a very useful predictor of mortality in COVID-19 patients.
This study has shown the need for monitoring the HDRs, in addition to the absolute cell counts, following COVID-19 diagnosis/during hospitalization of patients, and the utility of NLR and SII as predictors of mortality, but the study is not without its limitations. Besides being a retrospective study, analyzing the effect of co-morbidities on the hemogram would be important, though this has been widely studied. Also, intubation was used as the criterion for classifying the disease severity in this study, instead of the recommended criteria of severity classification, which should have been performed at diagnosis/presentation by the attending clinicians, but was not clearly stated in the medical records for all patients. Approximately forty-five percent of our patients did not have complete series of weekly hemograms and HDRs. This may have had a serious impact on our results, but with the general linear models used, these were excluded during analysis. Future prospective studies could help to explain some ambiguities in some of the associations found in this study. Furthermore, studying the effects of genetic variations (both viral and host) on the hemogram would be very useful in understanding the pathophysiology and management of COVID-19.
ConclusionThe non-survivors were older, had higher HDRs, and lower lymphocyte and red cell counts at COVID-19 diagnosis. The HDRs significantly decreased as the survivors recovered from the SARS-CoV-2 infection. While neutrophilia and lymphopenia improved in the survivor, they worsened in the non-survivor. These cells may have contributed to the recovery in the survivors by ameliorating the inflammatory response via clearance of the virus and to the death in the non-survivors by worsening the response via lung tissue damage and multi-organ dysfunction. Additionally, it is suggested that lower hematocrit and hemoglobin are associated with mortality in COVID-19. Putting these together, this study shows that serial NLR, PLR, MLR, NPR and SII, in addition to the routine cell counts, could serve as good, easily accessible and reliable markers of disease severity and predictors of mortality in patients with COVID-19 and could be used for the monitoring of the response to treatment.
The authors acknowledge the Teams of the Laboratory of Emerging Diseases (especially Barbara Fonseca Nogueira de Carvalho, Cintia Heloisa Correa Pilenso, Emerson Salvador de Souza França, Jessyka Farrah Fernandes Campos, José Luiz Rosenberis Cunha Junior, Kamila Cristina Silva, Kelly Krempser and Tania Regina Zaccariotto) and the Hematology Laboratory, Clinical Pathology Laboratory and Clinical Hospital of UNICAMP for performing the COVID-19 diagnosis and patients hemograms, respectively. The authors thank the Team of the Statistical Advice Service, School of Medical Sciences, UNICAMP.