It is important to know if patients with hemoglobinopathy could be more susceptible to COVID-19.
ObjectiveAnalyze SARS-CoV-2 infection in pediatric patients with hemoglobinopathy.
MethodsUsing the online platforms LILACS, PUBMED and EMBASE, on 17- JUL-2020 a search was made for the terms COVID-19 and SARS-CoV-2 associated with “sickle cell”, “thalassemia” and “hemoglobinopathy”.
ResultsThere were 623 pediatric and adult patients with sickle cell disease (SCD) or beta thalassemia (BT) and COVID-19. Total mortality rate was 6.42%. No pediatric patient with BT has been described. So, our analysis focused on children and adolescents with SCD: there were 121 pediatric patients, one adolescent died, prophylactic anticoagulation was prescribed to six patients, 11.76% needed intensive care unit, blood transfusion was prescribed in 29.70%. Vaso-occlusive crisis (VOC) and acute chest syndrome (ACS) were the main clinical manifestations in SCD.
DiscussionPediatric patients with SCD and COVID-19 have a low mortality rate when compared to adults, although is higher than the global pediatric population with COVID-19 (0−0.67%). The comorbidities associated with age and the long-term complications inherent to hemoglobinopathies may contribute to the increased mortality outside the pediatric age group. In SCD the clinical manifestations, both in children and adults, are VOC and ACS, and there was increase in blood requirement. Pediatric SCD patients with COVID-19 need more intensive care unit than the global pediatric population (3.30%).
ConclusionDespite pediatric population with SCD needs more intensive care, the outcome after infection by COVID-19 is favorable.
In less than three months after the first case reported in China, the infection called coronavirus disease-2019 (COVID-19), caused by the new “Severe Acute Respiratory Syndrome Coronavirus 2” (SARS-CoV-2), was recognized by the World Health Organization (WHO) as a pandemic.1 This is the worst pandemic in the last 100 years and is still uncontrolled.2
The epidemiology of the disease showed a higher lethality among elderly patients, mainly with chronic diseases such as diabetes mellitus, obesity, hypertension and cardiovascular disease.3,4
Thus, it is important to know if patients with hematological diseases that are predisposed to altered immune responses secondary to the disease itself or to the treatment could be more susceptible to this new pathology and present a higher risk of death.5 This relationship proved to be true considering malignant hematological diseases.6
Considering the hematological diseases called benign, patients with sickle cell disease (SCD) have immunodeficiency7 related to the disease itself, continuous use of medications or complications inherent to the disease.8,9 Functional asplenia provides a greater risk of infections by encapsulated bacteria, however, there is no relation to the increase in infections caused by viruses.8,10,11 Iron overload, both in beta thalassemia and in SCD, favors oxidative stress and in thalassemia can result in chronic organ damage, such as adrenal insufficiency, which could lead to immunodeficiency and increased risk of infections.9
ObjectiveAs SARS-CoV-2 spread easily in the world, much remains unknown about this virus and the higher susceptibility to infection of the people with hemoglobinopathy. This review aims to analyze the behavior of SARS-CoV-2 infection in pediatric patients with hemoglobinopathy, based on data from scientific medical publications, comparing it with published data on adults with hemoglobinopathy.
MethodsSearchUsing the online platforms LILACS, PUBMED and EMBASE as a database, a search was made on July 17, 2020 for the term “COVID-19”, associated with “sickle cell”, “thalassemia” and “hemoglobinopathy”. To increase the number of publications found, the association of “SARS-CoV-2” with the same terms was also researched. The search returned 47 articles. Also included were an abstract presented at the European Hematology Association Congress 2020 (EHA25)12 and data from the Surveillance Epidemiology of Coronavirus (COVID-19), under Research Exclusion - SECURE-SCD Registry (after being allowed by the investigator team),13 bringing the total to 49 scientific documents.
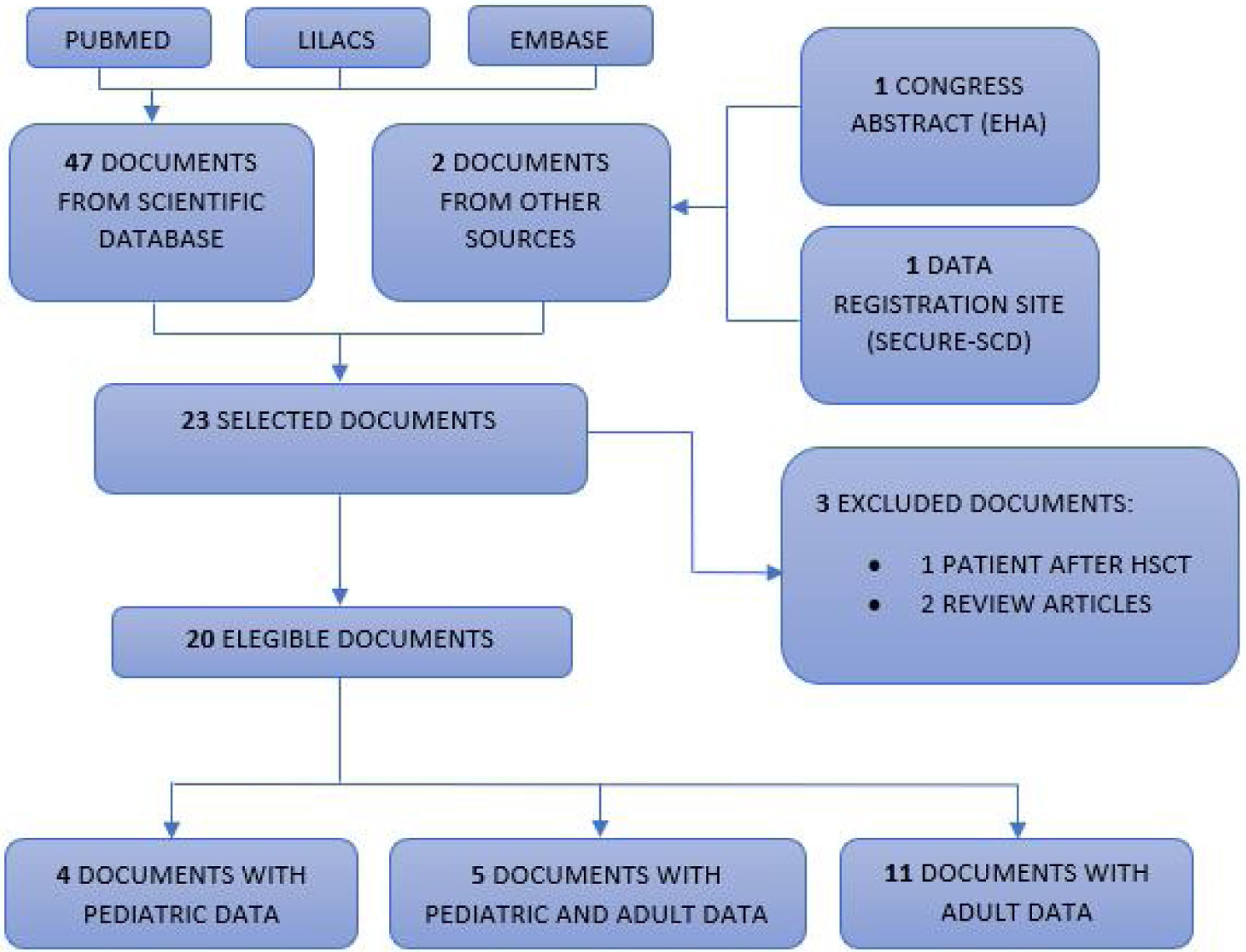
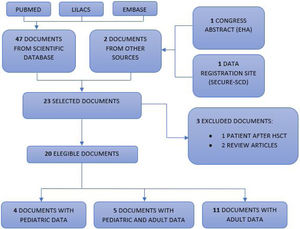
Selection of scientific documentsThe scientific documents were independently read by three researchers and then selected. Most of them were correspondences and letters to the editor. Of the 49 documents found, 26 were excluded because they did not present patient data and, therefore, 23 were selected.12–34 Of these, three more articles were excluded, as one was a report on a patient with sickle cell anemia after hematopoietic stem cell transplantation14 and two were published case reviews,15,16 totalizing 20 eligible scientific documents. Finally, four were exclusively pediatric descriptions (between zero and < 19 years old),17–20 five described both pediatric and adult data12,13,21–23 and 11 exclusively reported on adults.24–34Fig. 1 shows the selection of scientific documents.
PRISMA flow diagram.
Flowchart of publications included in this review. Our database searches identified a total of 47 unique records for the initial screening of abstracts and two documents from another source (congress summary and SECURE-SCD website), of which 20 were selected for full-text screening. Subsequently, three studies were excluded. Four pediatric articles and five articles with data on the pediatric population were included, totalizing 121 pediatric patients with hemoglobinopathies and COVID-19.
Data on pediatric patients with hemoglobinopathy and COVID-19 are presented in Table 1, grouped pediatric and adult patients in Table 2 and adults in Table 3.
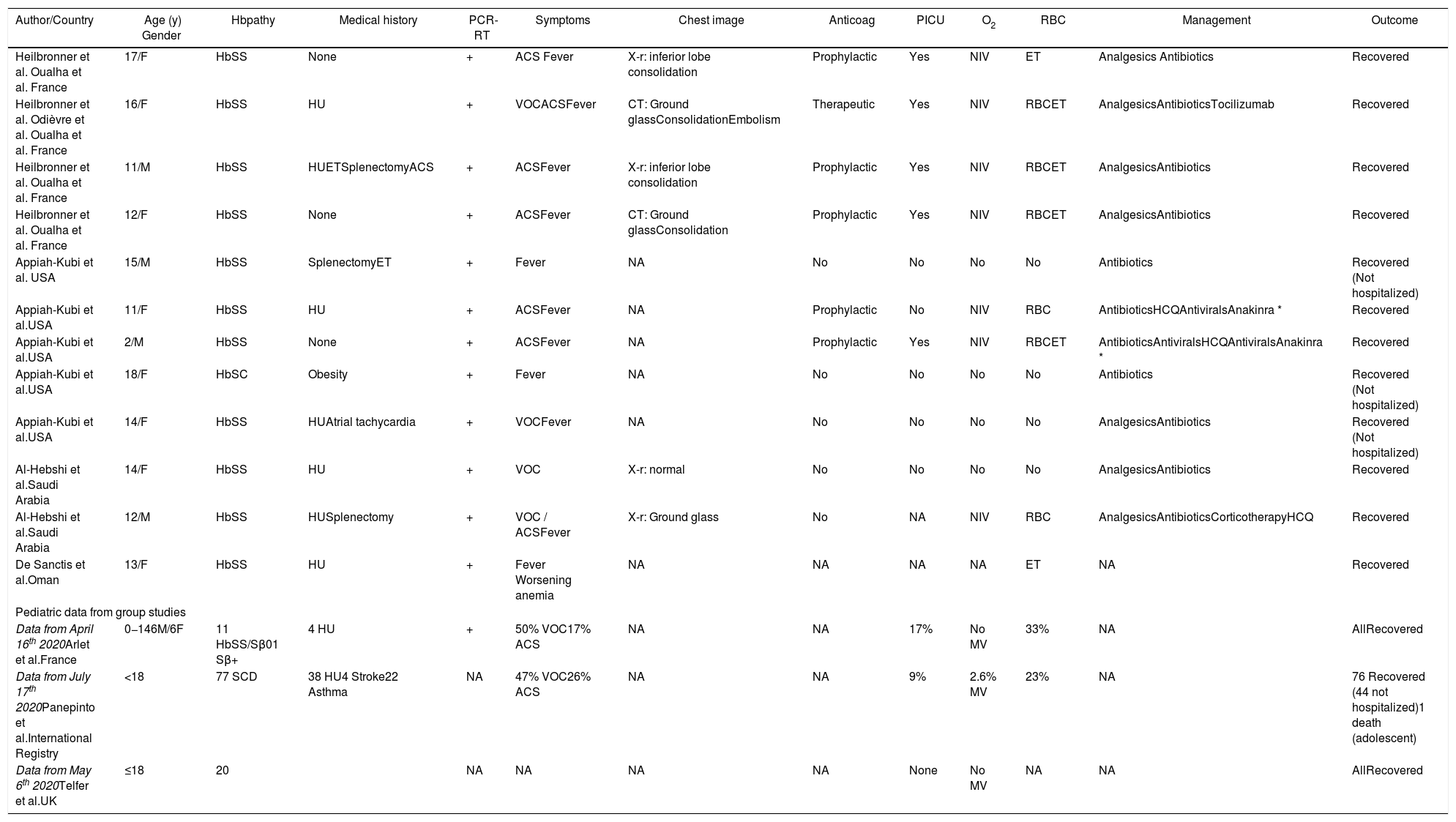
Summary data from pediatric patients with sickle cell disease and COVID-19 published in the literature.
| Author/Country | Age (y) Gender | Hbpathy | Medical history | PCR-RT | Symptoms | Chest image | Anticoag | PICU | O2 | RBC | Management | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heilbronner et al. Oualha et al. France | 17/F | HbSS | None | + | ACS Fever | X-r: inferior lobe consolidation | Prophylactic | Yes | NIV | ET | Analgesics Antibiotics | Recovered |
| Heilbronner et al. Odièvre et al. Oualha et al. France | 16/F | HbSS | HU | + | VOCACSFever | CT: Ground glassConsolidationEmbolism | Therapeutic | Yes | NIV | RBCET | AnalgesicsAntibioticsTocilizumab | Recovered |
| Heilbronner et al. Oualha et al. France | 11/M | HbSS | HUETSplenectomyACS | + | ACSFever | X-r: inferior lobe consolidation | Prophylactic | Yes | NIV | RBCET | AnalgesicsAntibiotics | Recovered |
| Heilbronner et al. Oualha et al. France | 12/F | HbSS | None | + | ACSFever | CT: Ground glassConsolidation | Prophylactic | Yes | NIV | RBCET | AnalgesicsAntibiotics | Recovered |
| Appiah-Kubi et al. USA | 15/M | HbSS | SplenectomyET | + | Fever | NA | No | No | No | No | Antibiotics | Recovered (Not hospitalized) |
| Appiah-Kubi et al.USA | 11/F | HbSS | HU | + | ACSFever | NA | Prophylactic | No | NIV | RBC | AntibioticsHCQAntiviralsAnakinra * | Recovered |
| Appiah-Kubi et al.USA | 2/M | HbSS | None | + | ACSFever | NA | Prophylactic | Yes | NIV | RBCET | AntibioticsAntiviralsHCQAntiviralsAnakinra * | Recovered |
| Appiah-Kubi et al.USA | 18/F | HbSC | Obesity | + | Fever | NA | No | No | No | No | Antibiotics | Recovered (Not hospitalized) |
| Appiah-Kubi et al.USA | 14/F | HbSS | HUAtrial tachycardia | + | VOCFever | NA | No | No | No | No | AnalgesicsAntibiotics | Recovered (Not hospitalized) |
| Al-Hebshi et al.Saudi Arabia | 14/F | HbSS | HU | + | VOC | X-r: normal | No | No | No | No | AnalgesicsAntibiotics | Recovered |
| Al-Hebshi et al.Saudi Arabia | 12/M | HbSS | HUSplenectomy | + | VOC / ACSFever | X-r: Ground glass | No | NA | NIV | RBC | AnalgesicsAntibioticsCorticotherapyHCQ | Recovered |
| De Sanctis et al.Oman | 13/F | HbSS | HU | + | Fever Worsening anemia | NA | NA | NA | NA | ET | NA | Recovered |
| Pediatric data from group studies | ||||||||||||
| Data from April 16th 2020Arlet et al.France | 0−146M/6F | 11 HbSS/Sβ01 Sβ+ | 4 HU | + | 50% VOC17% ACS | NA | NA | 17% | No MV | 33% | NA | AllRecovered |
| Data from July 17th 2020Panepinto et al.International Registry | <18 | 77 SCD | 38 HU4 Stroke22 Asthma | NA | 47% VOC26% ACS | NA | NA | 9% | 2.6% MV | 23% | NA | 76 Recovered (44 not hospitalized)1 death (adolescent) |
| Data from May 6th 2020Telfer et al.UK | ≤18 | 20 | NA | NA | NA | NA | None | No MV | NA | NA | AllRecovered | |
ACS, acute chest syndrome; Anticoag, anticoagulation; CT, computerized tomography; ET, exsanguineo transfusion; F, female; Hbpathy, hemoglobinopathy; HbSβ0, sickle cell disease Sβ0; HbSβ+, sickle cell disease Sβ+; HbSC, sickle cell disease SC; HbSS, sickle cell anemia; HCQ, hydroxychloroquine; HU, hydroxiurea; M, male; MV, mechanical ventilation; NA: Not available; NIV, non invasive ventilation; O2, oxygen; PCR-RT, SARS-CoV2 reverse-transcriptase polymerase-chain-reaction; PICU, Pediatric Intensive Care Unit; RBC, red blood cell transfusion; VOC, vaso-oclusive crisis; X-r, X-ray; y, years; +, positive.
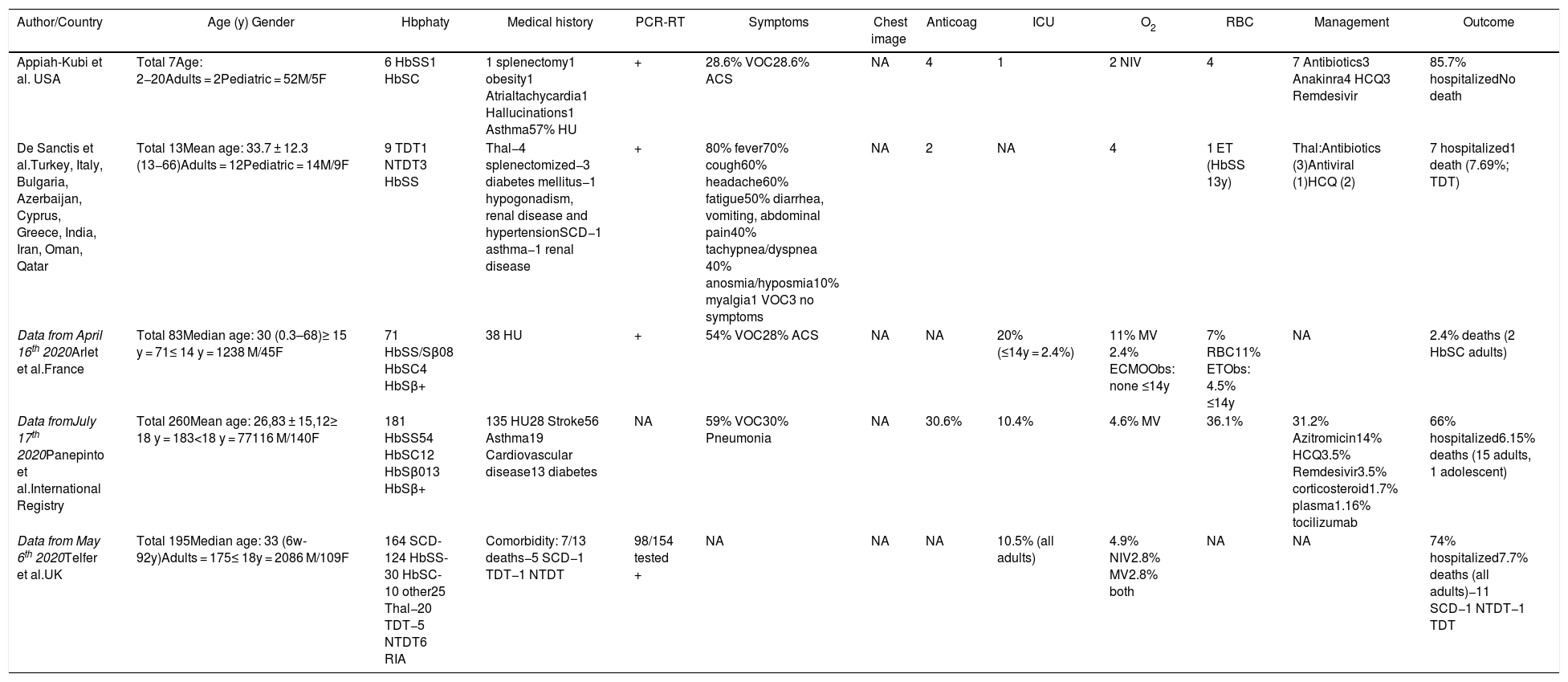
Summary data from pediatric and adult patients with hemoglobinopathy and COVID-19 described in group published in the literature.
| Author/Country | Age (y) Gender | Hbphaty | Medical history | PCR-RT | Symptoms | Chest image | Anticoag | ICU | O2 | RBC | Management | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appiah-Kubi et al. USA | Total 7Age: 2−20Adults = 2Pediatric = 52M/5F | 6 HbSS1 HbSC | 1 splenectomy1 obesity1 Atrialtachycardia1 Hallucinations1 Asthma57% HU | + | 28.6% VOC28.6% ACS | NA | 4 | 1 | 2 NIV | 4 | 7 Antibiotics3 Anakinra4 HCQ3 Remdesivir | 85.7% hospitalizedNo death |
| De Sanctis et al.Turkey, Italy, Bulgaria, Azerbaijan, Cyprus, Greece, India, Iran, Oman, Qatar | Total 13Mean age: 33.7 ± 12.3 (13−66)Adults = 12Pediatric = 14M/9F | 9 TDT1 NTDT3 HbSS | Thal−4 splenectomized−3 diabetes mellitus−1 hypogonadism, renal disease and hypertensionSCD−1 asthma−1 renal disease | + | 80% fever70% cough60% headache60% fatigue50% diarrhea, vomiting, abdominal pain40% tachypnea/dyspnea 40% anosmia/hyposmia10% myalgia1 VOC3 no symptoms | NA | 2 | NA | 4 | 1 ET (HbSS 13y) | Thal:Antibiotics (3)Antiviral (1)HCQ (2) | 7 hospitalized1 death (7.69%; TDT) |
| Data from April 16th 2020Arlet et al.France | Total 83Median age: 30 (0.3–68)≥ 15 y = 71≤ 14 y = 1238 M/45F | 71 HbSS/Sβ08 HbSC4 HbSβ+ | 38 HU | + | 54% VOC28% ACS | NA | NA | 20% (≤14y = 2.4%) | 11% MV 2.4% ECMOObs: none ≤14y | 7% RBC11% ETObs: 4.5% ≤14y | NA | 2.4% deaths (2 HbSC adults) |
| Data fromJuly 17th 2020Panepinto et al.International Registry | Total 260Mean age: 26,83 ± 15,12≥ 18 y = 183<18 y = 77116 M/140F | 181 HbSS54 HbSC12 HbSβ013 HbSβ+ | 135 HU28 Stroke56 Asthma19 Cardiovascular disease13 diabetes | NA | 59% VOC30% Pneumonia | NA | 30.6% | 10.4% | 4.6% MV | 36.1% | 31.2% Azitromicin14% HCQ3.5% Remdesivir3.5% corticosteroid1.7% plasma1.16% tocilizumab | 66% hospitalized6.15% deaths (15 adults, 1 adolescent) |
| Data from May 6th 2020Telfer et al.UK | Total 195Median age: 33 (6w-92y)Adults = 175≤ 18y = 2086 M/109F | 164 SCD- 124 HbSS- 30 HbSC- 10 other25 Thal−20 TDT−5 NTDT6 RIA | Comorbidity: 7/13 deaths−5 SCD−1 TDT−1 NTDT | 98/154 tested + | NA | NA | NA | 10.5% (all adults) | 4.9% NIV2.8% MV2.8% both | NA | NA | 74% hospitalized7.7% deaths (all adults)−11 SCD−1 NTDT−1 TDT |
ACS, acute chest syndrome; Anticoag, anticoagulation; ECMO, Extracorporeal Membrane Oxygenation; ET, exsanguineo transfusion; F, female; Hbpathy, hemoglobinopathy; HbSβ0, sickle cell disease Sβ0; HbSβ+, sickle cell disease Sβ+; HbSC, sickle cell disease SC; HbSS, sickle cell anemia; HCQ, hydroxychloroquine; HU, hydroxiurea; ICU, Intensive Care Unit; M, male; MV, mechanical ventilation; NA: Not available; NIV, non invasive ventilation; NTDT, non transfusion dependente talassemia; O2, oxygen; PCR-RT, SARS-CoV2 reverse-transcriptase polymerase-chain-reaction; RBC, red blood cell transfusion; RIA, rare inherited anemias; TDT, transfusion dependente talassemia; Thal, thalassemia; VOC, vaso-oclusive crisis; y, years; +, positive.
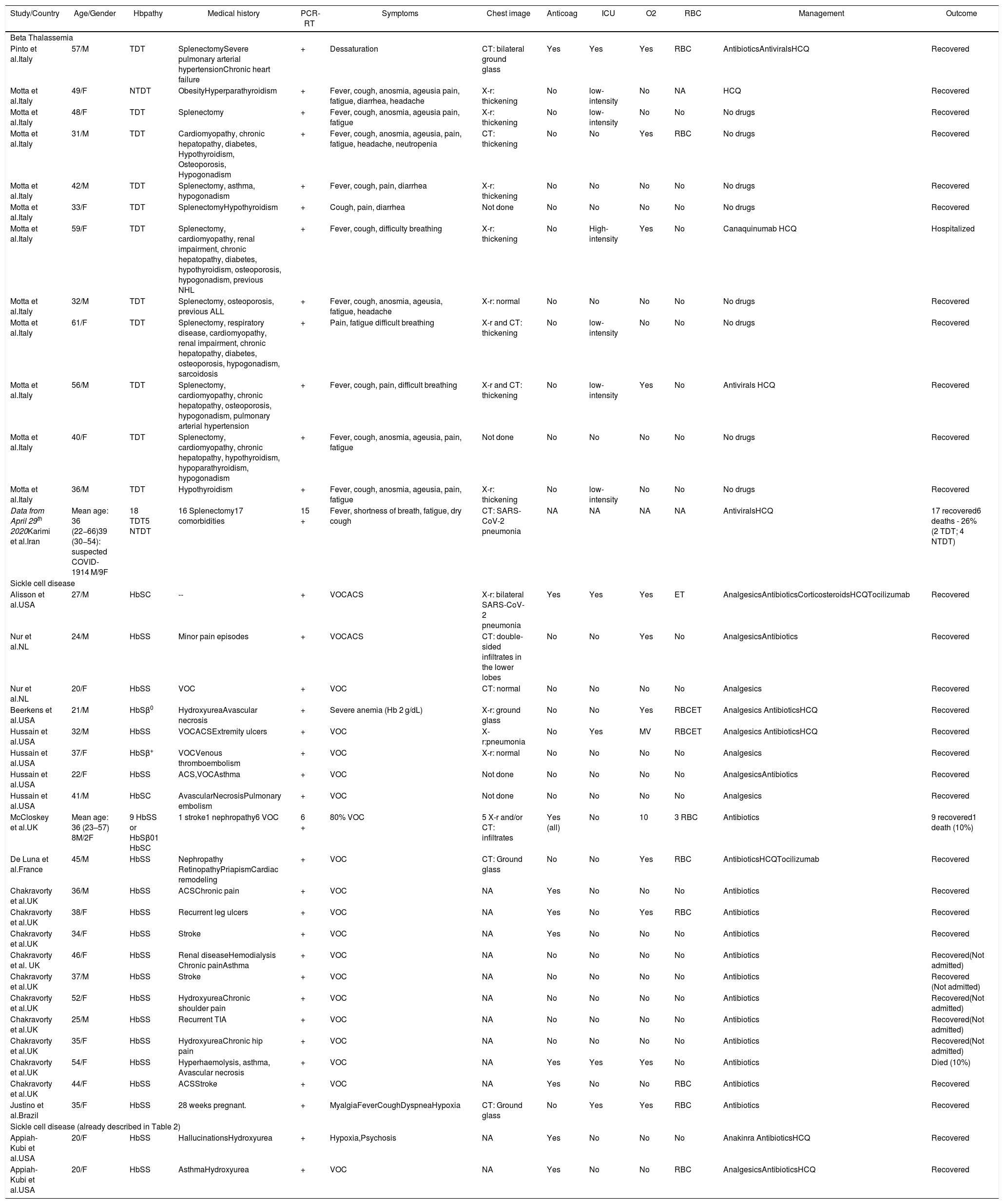
Summary data from adult patients with hemoglobinopathy and COVID-19 published in the literature.
| Study/Country | Age/Gender | Hbpathy | Medical history | PCR-RT | Symptoms | Chest image | Anticoag | ICU | O2 | RBC | Management | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta Thalassemia | ||||||||||||
| Pinto et al.Italy | 57/M | TDT | SplenectomySevere pulmonary arterial hypertensionChronic heart failure | + | Dessaturation | CT: bilateral ground glass | Yes | Yes | Yes | RBC | AntibioticsAntiviralsHCQ | Recovered |
| Motta et al.Italy | 49/F | NTDT | ObesityHyperparathyroidism | + | Fever, cough, anosmia, ageusia pain, fatigue, diarrhea, headache | X-r: thickening | No | low-intensity | No | NA | HCQ | Recovered |
| Motta et al.Italy | 48/F | TDT | Splenectomy | + | Fever, cough, anosmia, ageusia pain, fatigue | X-r: thickening | No | low-intensity | No | No | No drugs | Recovered |
| Motta et al.Italy | 31/M | TDT | Cardiomyopathy, chronic hepatopathy, diabetes, Hypothyroidism, Osteoporosis, Hypogonadism | + | Fever, cough, anosmia, ageusia, pain, fatigue, headache, neutropenia | CT: thickening | No | No | Yes | RBC | No drugs | Recovered |
| Motta et al.Italy | 42/M | TDT | Splenectomy, asthma, hypogonadism | + | Fever, cough, pain, diarrhea | X-r: thickening | No | No | No | No | No drugs | Recovered |
| Motta et al.Italy | 33/F | TDT | SplenectomyHypothyroidism | + | Cough, pain, diarrhea | Not done | No | No | No | No | No drugs | Recovered |
| Motta et al.Italy | 59/F | TDT | Splenectomy, cardiomyopathy, renal impairment, chronic hepatopathy, diabetes, hypothyroidism, osteoporosis, hypogonadism, previous NHL | + | Fever, cough, difficulty breathing | X-r: thickening | No | High-intensity | Yes | No | Canaquinumab HCQ | Hospitalized |
| Motta et al.Italy | 32/M | TDT | Splenectomy, osteoporosis, previous ALL | + | Fever, cough, anosmia, ageusia, fatigue, headache | X-r: normal | No | No | No | No | No drugs | Recovered |
| Motta et al.Italy | 61/F | TDT | Splenectomy, respiratory disease, cardiomyopathy, renal impairment, chronic hepatopathy, diabetes, osteoporosis, hypogonadism, sarcoidosis | + | Pain, fatigue difficult breathing | X-r and CT: thickening | No | low-intensity | No | No | No drugs | Recovered |
| Motta et al.Italy | 56/M | TDT | Splenectomy, cardiomyopathy, chronic hepatopathy, osteoporosis, hypogonadism, pulmonary arterial hypertension | + | Fever, cough, pain, difficult breathing | X-r and CT: thickening | No | low-intensity | Yes | No | Antivirals HCQ | Recovered |
| Motta et al.Italy | 40/F | TDT | Splenectomy, cardiomyopathy, chronic hepatopathy, hypothyroidism, hypoparathyroidism, hypogonadism | + | Fever, cough, anosmia, ageusia, pain, fatigue | Not done | No | No | No | No | No drugs | Recovered |
| Motta et al.Italy | 36/M | TDT | Hypothyroidism | + | Fever, cough, anosmia, ageusia, pain, fatigue | X-r: thickening | No | low-intensity | No | No | No drugs | Recovered |
| Data from April 29th 2020Karimi et al.Iran | Mean age: 36 (22−66)39 (30−54): suspected COVID-1914 M/9F | 18 TDT5 NTDT | 16 Splenectomy17 comorbidities | 15 + | Fever, shortness of breath, fatigue, dry cough | CT: SARS-CoV-2 pneumonia | NA | NA | NA | NA | AntiviralsHCQ | 17 recovered6 deaths - 26% (2 TDT; 4 NTDT) |
| Sickle cell disease | ||||||||||||
| Alisson et al.USA | 27/M | HbSC | -- | + | VOCACS | X-r: bilateral SARS-CoV-2 pneumonia | Yes | Yes | Yes | ET | AnalgesicsAntibioticsCorticosteroidsHCQTocilizumab | Recovered |
| Nur et al.NL | 24/M | HbSS | Minor pain episodes | + | VOCACS | CT: double-sided infiltrates in the lower lobes | No | No | Yes | No | AnalgesicsAntibiotics | Recovered |
| Nur et al.NL | 20/F | HbSS | VOC | + | VOC | CT: normal | No | No | No | No | Analgesics | Recovered |
| Beerkens et al.USA | 21/M | HbSβ0 | HydroxyureaAvascular necrosis | + | Severe anemia (Hb 2 g/dL) | X-r: ground glass | No | No | Yes | RBCET | Analgesics AntibioticsHCQ | Recovered |
| Hussain et al.USA | 32/M | HbSS | VOCACSExtremity ulcers | + | VOC | X-r:pneumonia | No | Yes | MV | RBCET | Analgesics AntibioticsHCQ | Recovered |
| Hussain et al.USA | 37/F | HbSβ+ | VOCVenous thromboembolism | + | VOC | X-r: normal | No | No | No | No | Analgesics | Recovered |
| Hussain et al.USA | 22/F | HbSS | ACS,VOCAsthma | + | VOC | Not done | No | No | No | No | AnalgesicsAntibiotics | Recovered |
| Hussain et al.USA | 41/M | HbSC | AvascularNecrosisPulmonary embolism | + | VOC | Not done | No | No | No | No | Analgesics | Recovered |
| McCloskey et al.UK | Mean age: 36 (23–57) 8M/2F | 9 HbSS or HbSβ01 HbSC | 1 stroke1 nephropathy6 VOC | 6 + | 80% VOC | 5 X-r and/or CT: infiltrates | Yes (all) | No | 10 | 3 RBC | Antibiotics | 9 recovered1 death (10%) |
| De Luna et al.France | 45/M | HbSS | Nephropathy RetinopathyPriapismCardiac remodeling | + | VOC | CT: Ground glass | No | No | Yes | RBC | AntibioticsHCQTocilizumab | Recovered |
| Chakravorty et al.UK | 36/M | HbSS | ACSChronic pain | + | VOC | NA | Yes | No | No | No | Antibiotics | Recovered |
| Chakravorty et al.UK | 38/F | HbSS | Recurrent leg ulcers | + | VOC | NA | Yes | No | Yes | RBC | Antibiotics | Recovered |
| Chakravorty et al.UK | 34/F | HbSS | Stroke | + | VOC | NA | Yes | No | No | No | Antibiotics | Recovered |
| Chakravorty et al. UK | 46/F | HbSS | Renal diseaseHemodialysis Chronic painAsthma | + | VOC | NA | No | No | No | No | Antibiotics | Recovered(Not admitted) |
| Chakravorty et al.UK | 37/M | HbSS | Stroke | + | VOC | NA | No | No | No | No | Antibiotics | Recovered (Not admitted) |
| Chakravorty et al.UK | 52/F | HbSS | HydroxyureaChronic shoulder pain | + | VOC | NA | No | No | No | No | Antibiotics | Recovered(Not admitted) |
| Chakravorty et al.UK | 25/M | HbSS | Recurrent TIA | + | VOC | NA | No | No | No | No | Antibiotics | Recovered(Not admitted) |
| Chakravorty et al.UK | 35/F | HbSS | HydroxyureaChronic hip pain | + | VOC | NA | No | No | No | No | Antibiotics | Recovered(Not admitted) |
| Chakravorty et al.UK | 54/F | HbSS | Hyperhaemolysis, asthma, Avascular necrosis | + | VOC | NA | Yes | Yes | Yes | No | Antibiotics | Died (10%) |
| Chakravorty et al.UK | 44/F | HbSS | ACSStroke | + | VOC | NA | Yes | No | No | RBC | Antibiotics | Recovered |
| Justino et al.Brazil | 35/F | HbSS | 28 weeks pregnant. | + | MyalgiaFeverCoughDyspneaHypoxia | CT: Ground glass | No | Yes | Yes | RBC | Antibiotics | Recovered |
| Sickle cell disease (already described in Table 2) | ||||||||||||
| Appiah-Kubi et al.USA | 20/F | HbSS | HallucinationsHydroxyurea | + | Hypoxia,Psychosis | NA | Yes | No | No | No | Anakinra AntibioticsHCQ | Recovered |
| Appiah-Kubi et al.USA | 20/F | HbSS | AsthmaHydroxyurea | + | VOC | NA | Yes | No | No | RBC | AnalgesicsAntibioticsHCQ | Recovered |
ACS, acute chest syndrome; ALL, acute lymphocytic leucemia; Anticoag, anticoagulation; ET, exsanguineo transfusion; F, female; Hbpathy, hemoglobinopathy; HbSβ0, sickle cell disease Sβ0; HbSβ+, sickle cell disease Sβ+; HbSC, sickle cell disease SC; HbSS, sickle cell anemia; HCQ, hydroxychloroquine; ICU, Intensive Care Unit; M, male; MV, mechanical ventilation; NA: Not available; NHL, non-Hodgkin lymphoma; NTDT, non transfusion dependente talassemia; O2, oxygen; PCR-RT, SARS-CoV2 reverse-transcriptase polymerase-chain-reaction; RBC, red blood cell transfusion; TDT, transfusion dependente talassemia; Thal, thalassemia; TIA, transient ischemic attack; VOC, vaso-oclusive crisis; X-r, X-ray; y, years; +, positive.
We excluded seven patients (one sickle cell trait and six rare inherited anemias) from this analysis. Hence, there are 623 pediatric and adult patients with hemoglobinopathy (SCD or beta thalassemia) and COVID-19 in the 20 selected documents. SCD was present in 553 patients (88.76%) and beta-thalassemia in 70 (11.24%). The total mortality rate in this review was 6.42% (40/623). The mortality rate in SCD ranged from 0% to 10% and in beta thalassemia between 0% and 26%, according to the evaluated report. A total of 12.82% (75/585) patients needed the intensive care unit during hospitalization and 47 advanced oxygen supply, being 30 by mechanical ventilation under orotracheal intubation (including two patients treated with extracorporeal membrane oxygenation), and 17 with non-invasive ventilation. Red blood cell transfusion (RBC) or exchange transfusion was performed in 35.57% (148/416) of the patients (Tables 1, 2 and 3).
Considering available pediatric data (Table 1), there are 121 patients, representing 19.42% of all patients. At the pediatric age, only one adolescent with SCD died (0.82% of the pediatric population). The main clinical manifestation at hospital admission or during hospitalization was the vaso-occlusive crisis (VOC), followed by the acute chest syndrome (ACS). A total of 41 pediatric patients had VOC, 27 ACS and two both VOC and ACS. Prophylactic anticoagulation was prescribed to six patients at two medical centers, with one report of pulmonary thromboembolism. There was a need in 14 of 119 patients for the pediatric intensive care unit (11.76%), with seven under advanced oxygen supply and two with mechanical ventilation by orotracheal intubation (1.68%). RBC or exchange transfusions were performed in 30 of 101 patients with accessible data (29.70%). An adolescent patient received tocilizumab and two patients Anakinra for cytokine storm syndrome, all with favorable outcomes. It is also worth mentioning that 47 (38.84%) of the pediatric patients were not hospitalized.
In Tables 2 and 3, the VOC was also the main clinical manifestation in SCD adult patients at hospital admission or during hospitalization, also followed by the ACS. There were no registries of children and adolescents with beta thalassemia and COVID-19 in this review.
DiscussionThe emergence of a rapidly spreading viral disease around the world, as in 2009 with the H1N1 Influenza virus, is of great concern among patients with chronic pathologies. During the H1N1 outbreak, 50% of the SCD pediatric cases with H1N1 went to the hospital and 25% developed ACS.28,35 Therefore, a new virus with a high risk for respiratory complications in adult and elderly patients and which, unlike H1N1, does not have a developed available vaccine, has a catastrophic potential, especially in the poorest regions of the planet.36
As there were no reports of pediatric patients with thalassemia, our analysis focused on children and adolescents with SCD. When assessing mortality in pediatric and adult patients with hemoglobinopathy, a higher percentage was found when compared to the general population (estimated at 4.30% on July 17th 2020 according to WHO)..37 Fortunately, in pediatric patients there has been a low mortality rate, with one case of an adolescent observed in this review. The presence of comorbidities associated with age,3,4,38 as well as the known long-term complications inherent to hemoglobinopathies, may contribute to the increased mortality out of the pediatric age group bracket.39,40 The clinical course of COVID-19 in pediatric patients has been favorable, but data on children and adolescents with chronic diseases are still scarce.41
In children and adolescents with SCD and COVID-19, the presence of VOC and ACS were common at hospital admission or during hospitalization. It is known that these acute events are preceded in most cases by infection.42 The ACS is epidemiologically a complication of the VOC,43 having a complex pathophysiology and resulting in an acute lung injury indistinguishable from a multilobed pneumonia.44 The radiological evaluation by computed tomography shows consolidation in most cases, but the presence of the ground glass image, as well as commonly present in patients with COVID-19,45 appears in practically a quarter of the patients with ACS.46 In the absence of a positive real-time polymerase chain reaction (RT-PCR) test for SARS-CoV-2, the same appearance of the radiological image can make the diagnosis difficult.47 In contrast, in patients with SCD and confirmed SARS-CoV2 infection, the diagnosis of ACS may be underestimated.
Another important pathophysiological mechanism in the ACS is the presence of fat embolism and/or bone marrow embolism in the circulation.44 For this reason, some medical centers include prophylactic anticoagulation in ACS as an institutional protocol. This is the reality of one of the pediatric centers described in this review, where four patients received Low Molecular Weight Heparin (LMWH).17 In this report, one adolescent receiving prophylactic anticoagulation changed to a therapeutic dose after the chest computed tomography showed a pulmonary embolism. The other two patients on anticoagulation, from the six described in Table 1, received prophylactic doses, following thromboprophylaxis guidelines for COVID-19.20 In COVID-19, the presence of microvascular thrombosis, mainly pulmonary, was also observed, although its mechanism has not yet been clarified.48 This evidence resulted in the recommendation for anticoagulation for adult patients with COVID-19.49 However, there is no such evidence in pediatrics and furthermore, there is controversy regarding the need for anticoagulation, even if prophylactic, for all patients.50
Patients with hemoglobinopathy usually need transfusion therapy when hospitalized. Among patients with SCD, hemolysis intensifies in the presence of infectious processes51 and for patients with ACS, there is a suggestion to maintain hemoglobin levels stable at 9–11 g/dL or hemoglobin S levels below 30%.52,53 In this review, all seven SCD pediatric patients described individually in Table 1 who presented with ACS required RBC transfusion and/or exchange transfusion, with a good outcome after the procedure. Therefore, blood transfusion seems to rapidly improve oxygen saturation20,23 and it is possible that early and aggressive transfusion for ACS may be beneficial to COVID-19 patients.21
The clinical course of COVID-19 in pediatric patients with SCD requires some attention regarding the need for an intensive care unit, which seems to make no difference, when compared to adults with hemoglobinopathy. However, compared to global pediatric data, in which the average intensive care unit need is 3.30%,54 SCD pediatric patients seem to have a greater requirement for intensive care support. This is in line with the data that 83% of patients admitted to a pediatric intensive care unit have chronic diseases.55 In this review, advanced oxygen supply in pediatrics was relatively lower, when compared to adults, mainly in mechanical ventilation, corroborating the pediatric best outcome. Finally, mortality rate in pediatrics varied between 0−0.67%56 and in this review, for SCD pediatric patients, it was 0.82%, suggesting the need of further studies and case reports on pediatric patients with hemoglobinopathy for better understanding. Although we did not perform the statistical analysis to determine whether this difference in the severity of the disease is statistically significant, our hypothesis is that the underlying disease may be responsible for the need for more intensive care during hospitalization for any infection in patients with SCD. Thus, the greater need for the ICU can be explained by clinical manifestations, such as ACS, or by the comorbidities that the disease can cause.
The identification of possible duplicate data in a few case reports and reviews was considered a limitation of this review, making it difficult to accurately number the patients. All the data found were described in the Tables. Thus, we suggest to future authors that they specify in their articles whether the patient reported was part of a larger registry, such as the UK Haemoglobinopathy Coordinating Centres12 and the SECURE-SCD.57
In conclusion, the pediatric population with SCD needs more intensive care during hospitalization, but with a favorable outcome after infection by COVID-19. National and international registries of pediatric patients with hemoglobinopathy should be prioritized to obtain robust data on this population.
Conflicts of interestThe authors declare no conflicts of interest.
We thank Julie A. Panepinto, Department of Pediatrics, Medical College of Wisconsin, for making the SECURE-SCD data available.