Hemostatic abnormalities and thrombotic risk associated with coronavirus disease 2019 (COVID-19) are among the most discussed topics in the management of this disease. The aim of this position paper is to provide the opinion of Brazilian experts on the thromboprophylaxis and management of thrombotic events in patients with suspected COVID-19, in the sphere of healthcare in Brazil. To do so, the Brazilian Society of Thrombosis and Hemostasis (BSTH) and the Thrombosis and Hemostasis Committee of the Brazilian Association of Hematology, Hemotherapy and Cellular Therapy (ABHH) have constituted a panel of experts to carefully review and discuss the available evidence about this topic. The data discussed in this document was reviewed by May 9, 2020. Recommendations and suggestions reflect the opinion of the panel and should be reviewed periodically as new evidence emerges.
Coronavirus disease (COVID-19), previously known as 2019 novel coronavirus, is an acute respiratory infection caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), initially identified in China at the end of 2019.1 In Brazil, COVID-19 had been diagnosed in more than 150,000 patients and caused more than 10,000 deaths by May 9, 2020, according to official data from the Brazilian Ministry of Health.2
During the COVID-19 outbreak in China, it was observed that approximately 80% of the patients had mild to moderate upper respiratory tract symptoms, 13.8% had severe disease (dyspnea, tachypnea, and hypoxia), which required hospitalization, and 6.1% developed critical symptoms (respiratory failure, septic shock or multiple organ dysfunction) that required treatment in an intensive care unit (ICU).3 Severe symptoms usually appeared after approximately 7–8 days from the onset of the disease and progression to critical COVID-19 usually occurred after the 11th day of symptoms.4,5
The pathogenesis of COVID-19 is related to both the invasion of lung epithelial cells by SARS-CoV-2 and the host immune reaction against the virus.6 Uncontrolled systemic inflammatory response, resulting from the release of large amounts of pro-inflammatory cytokines is one of the hallmarks of the severe acute respiratory distress syndrome (ARDS) and multiple organ failure, the two main causes of death by COVID-19.6 Besides inflammation, COVID-19 patients may present with signs of hypercoagulability, characterized by pronounced elevation of fibrinogen levels and D-dimers, and may develop disseminated intravascular coagulation (DIC) at late stages of the disease.7,8 Increased D-dimer levels and the diagnosis of DIC are associated with a poor prognosis and death.8 Moreover, mounting evidence confirms that the incidence of arterial and venous thrombosis is increased in COVID-19 and that thrombotic events are associated with higher mortality.7 Therefore, the prevention of thrombosis is an essential part of the clinical management of these patients.
Overview of the evidence and use of this documentCOVID-19 is a new disease with an unprecedented impact on public health. Accordingly, an impressive amount of information is being incorporated in the medical literature, with heterogeneous quality and unknown generalizability. At the same time, clinical dilemmas involving the management of patients increased the expectation of practicing physicians for guidance from trusted sources.9 COVID-19-associated hemostatic abnormalities and thrombotic risk are among the most discussed topics in medical care of these patients and have raised several questions regarding optimal clinical management.10–13
Currently, due to the lack of high-quality studies and to the rapidly evolving literature, it is not possible to organize a formal medical guideline for these topics. However, we believe that providing the opinion of a panel of experts based on a careful and balanced review of the available evidence would be useful to the medical community in Brazil. Furthermore, healthcare in Brazil and available resources is rather heterogeneous and certainly differs from the reality in other countries, where other guidance papers are being published.10,14–16
In this context, the Brazilian Society of Thrombosis and Hemostasis (BSTH) and the Thrombosis and Hemostasis Committee of the Brazilian Association of Hematology, Hemotherapy and Cellular Therapy (ABHH) have constituted a panel of experts to discuss the current evidence available on the thromboprophylaxis and management of thrombotic events in patients with suspected COVID-19, in the sphere of healthcare in Brazil. The data discussed in this document was reviewed by May 9, 2020. Recommendations and suggestions reflect the opinion of the panel and should be reviewed periodically as new evidence emerges.
Role of disseminated intravascular coagulopathy in COVID-19The host response to pathogens and sepsis involves the activation of multiple systems among which are innate immunity and hemostasis. Both systems have been shown to contribute to pathogen eradication by facilitating the access and the microbicidal function of phagocytes.17 However, deregulation and/or loss of localization of this so-called immunothrombotic response can lead to secondary damage mediated by thrombus formation.18 Accordingly, laboratory alterations consistent with the activation of hemostasis and fibrinolysis, such as increased levels of D-dimer, prolongation of the prothrombin time (PT), high fibrinogen levels, as well as consumption of platelets and fibrinogen in more severe cases, can be observed in the course of several infections and should be regarded as part of the host response to pathogens.19 This is particularly evident in COVID-19 patients, in whom increased D-dimer levels (which parallel the increase of IL-6) has been identified as an independent marker of severity.6 In fact, the association between increased D-dimer levels and poor prognosis has also been described in other forms of sepsis and pneumonia.20,21
Of note, in a recent comparison of D-dimer levels between COVID-19 and non-COVID-19 pneumonia patients in China, similar levels were observed in both conditions.22 Similarly, the accumulation of fibrin in alveolar spaces and even the formation of microvascular thrombosis described in COVID-19, which has raised questions on whether anticoagulants should be used in higher doses in these patients, is also part of immunothrombosis and has been described in other forms of ARDS.23,24 Moreover, the use of several anticoagulant molecules was not capable of improving the outcome of sepsis in these patients.21,25–27
Therefore, until the benefit of using therapeutic doses of anticoagulants in patients with COVID-19 based solely on D-dimer levels (i.e., in the absence of highly suspected or proven venous thromboembolism [VTE]) is demonstrated by randomized clinical trials, the use of anticoagulants in COVID-19 should be restricted to prophylaxis of VTE or the treatment of confirmed VTE events. These include deep venous thrombosis (DVT) (both symptomatic and asymptomatic, detected by screening methods), pulmonary embolism (PE) and catheter-associated thrombosis.
Monitoring of coagulopathyHypercoagulability in COVID-19 is characterized by increased levels of fibrinogen and D-dimers, prolonged PT and activated partial thromboplastin time (aPTT), mild thrombocytopenia (100–150×109/L), elevated levels of factor VIII (FVIII) and Von Willebrand factor (VWF).4,6,26,28–30 In addition, signs of DIC have been reported in late stages of severe COVID-19.6–8
D-dimer levels are particularly high in critically ill patients in the ICU4,29 and among non-survivors,6 which suggests that D-dimer is a prognostic marker in COVID-19 and testing for it should be initially performed in all hospitalized patients. The association between increased D-dimers and severe COVID-19 may in part be explained by the above-mentioned interplay between inflammatory response and activation of coagulation. From that perspective, D-dimer levels can represent a surrogate marker for COVID-19 severity.8
This is not novel, as some authors have used an elevated D-dimer as part of as a scoring system to identify those at increased risk for VTE.31 Additionally, a recent Brazilian study showed that elevated D-dimer levels is common in viral infections in our country, such as Zika Virus and Chikungunya, which may be associated with an increase in the risk of thromboembolism.32 Lastly, D-dimers are elevated in the elderly, individuals with comorbidities, such as infection, inflammation, cancer, and the hospitalized patients. Nevertheless, to date, there is no validated cut-off value for D-dimer to guide changes in the management of anticoagulation in COVID-19 patients.
Therefore, considering the clinical impact of hypercoagulability in COVID-19, analysis of peripheral blood smear, platelet count, PT, aPTT, fibrinogen and D-dimer levels is recommended for all hospitalized patients. These parameters should be regularly monitored in critically ill patients. Increasing trends of D-dimer and other clinical variables, such as levels of oxygenation and ventilator parameters and possibly imaging studies, whenever feasible, must be jointly considered toward the suspicion of thrombotic events and the need for full anticoagulation. The most adequate time interval between tests is uncertain and testing should be based on clinical indication, assays availability and the local laboratory capacity and facilities.
Systematic venous thromboembolism assessmentThe reported in-hospital cumulative incidence of VTE in COVID-19 patients ranges from 8 to 69%,7,33–36 with the highest incidence among patients in the ICU. Discrepancies in VTE incidence among studies can be attributed to differences in population, disease severity, strategies of thromboprophylaxis and practices regarding the performance of imaging tests. It is noteworthy that about half of the VTE patients were asymptomatic in one cohort from the Netherlands35 and the highest incidence of VTE (69%) was reported by a French study in which systematic ultrasounds were performed.36
Based on concerns of the increased risk of VTE and the importance of early VTE detection, some experts and medical societies have recommended screening for DVT in COVID-19 patients in the ICU at admission and every 4–5 days thereafter.12,35 In this context, duplex scan is the imaging test of choice because it is easy to perform and almost risk-free. Furthermore, it can be performed at bedside, without the need to remove the patient from the ICU. Special attention should be given to avoid exposure of the medical team while performing the tests and to prevent unnecessary transport of more severe patients out of the isolation units.
Thus, we suggest performing venous compression Duplex scan in ICU patients at admission and then at regular intervals (ideally every 4–5 days), whenever available or guided by clinical suspicion, to detect DVT and to prevent its complications. We also suggest maintaining vigilance for clinical and echocardiographic signs of PE,37 given that PE seems to be more frequent than DVT in COVID-19 patients. Routine screening for VTE in hospitalized patients with COVID-19, solely based on elevated D-Dimer, cannot be recommended at this point.
Prophylaxis of venous thromboembolismIndication of thromboprophylaxisRisk-assessment of VTE is recommended for all surgical and medical patients admitted to a hospital and those with acute respiratory and infectious diseases are at high risk of hospital-associated VTE, according to international guidelines.38–40 Several Brazilian hospitals have implemented systematic VTE risk-assessment protocols to the routine medical care as part of quality improvement measures and different VTE risk-assessment tools are available, such as IMPROVE score,41 the Brazilian Guideline for VTE Prophylaxis,42 the Caprini score43 and the Padua score.44
However, the risk of hospital-associated VTE seems to be higher in hospitalized patients with COVID-19 than in other medical patients.7 It was demonstrated that 40% of patients hospitalized for COVID-19 (407/1026) present with a high Padua score (greater than or equal to 4) upon admission45 and without thromboprophylaxis a great proportion of patients may develop VTE, as shown in a Chinese cohort of severe COVID-19 pneumonia in which 25% of the patients had DVT of the lower limbs during hospitalization.46 These observations suggest that the risk for thrombosis in COVID-19 is a result not only of respiratory distress and acute infectious disease, but also of the disease associated coagulopathy.
Despite the highest risk of thrombosis being reported among patients in the ICU, a high incidence of VTE has also been observed in those admitted to general wards.33,35 The reported cumulative incidence of VTE in patients with COVID-19 during hospitalization in general wards ranges from 6% to 9%,33,35 of which half of the thrombotic events have been diagnosed in the first 24h of hospitalization33 and 56% of the patients were not receiving thromboprophylaxis.33 Moreover, VTE was independently associated with death (hazard ratio (HR) of death, adjusted for age, sex and ICU stay, 2.4; 95% CI, 1.02–5.5) in a Dutch cohort.35
Therefore, we recommend that all patients hospitalized for suspected or confirmed COVID-19 should receive pharmacologic thromboprophylaxis, in the absence of absolute contraindications. We suggest the use of low molecular weight heparin (LMWH) due to the ease of use and once daily dosing regimen. Alternatively, unfractionated heparin (UFH) or fondaparinux (particularly for patients with heparin-induced thrombocytopenia) can be given for thromboprophylaxis. In case a pharmacologic thromboprophylaxis is contraindicated, mechanical prophylaxis should be used as an alternative. Table 1 contains the contraindications for pharmacologic thromboprophylaxis in hospitalized medical patients.
Risk factors for bleeding that contraindicate pharmacologic thromboprophylaxis.
| 1. Active bleeding | 6. Acute stroke |
| 2. Acquired bleeding disorders | 7. Thrombocytopenia<25×109/L (*) |
| 3. Concurrent use of anticoagulants | 8. Uncontrolled systolic hypertension |
| 4. Lumbar puncture/epidural/spinal anesthesia expected within the next 12h | 9. Untreated inherited bleeding disorders |
| 5. Lumbar puncture/epidural/spinal anesthesia within the previous 4h |
Adapted from Nice Clinical Guidelines 92 – Venous thromboembolism. 2010 (https://www.acutemedicine.org.uk/wp-content/uploads/2015/12/NICE-Reducing-VTE-201015.pdf) and (*) from The ASH COVID Resources. 2020 (https://www.hematology.org/covid-19/covid-19-and-coagulopathy).
Among severe COVID-19 patients admitted to ICU, the cumulative incidence of in-hospital thrombotic events ranges from 40 to 60%,7,34–36 despite the use of pharmacologic thromboprophylaxis. The most frequently diagnosed thrombotic complication has been PE.7,33–35
Based on this observation, some authors and medical societies advocate the use of intermediate or therapeutic doses of LMWH for VTE prophylaxis in ICU patients with COVID-19.7,11,12,34,35 The dosing regimens that have been proposed are enoxaparin 40mg twice daily,12 enoxaparin 1mg/kg daily,11 therapeutic doses of LMWH (type of LMWH is not specified),33,36 nadroparin 5700IU daily34 and nadroparin 2850IU twice daily (body weight<100kg).35 Of note, thrombotic events were also diagnosed in patients who were already receiving intermediate or therapeutic doses of LMWH.33,35,36 Middeldorp et al. further reported that the risk of VTE in ICU patients with COVID-19 was similar in the period when the standard dose of nadroparin prophylaxis was doubled (58%) in comparison with the period when standard prophylaxis was used (41%).35 Although thrombotic risk is high in severe COVID-19, possibly higher than in non-COVID-19 critically ill patients,7 there is no evidence to date supporting the increase of the dose of pharmacologic thromboprophylaxis to intermediate, nor therapeutic doses. Nevertheless, dose adjustments of LMWH and UFH for VTE prophylaxis have been suggested for obese surgical and medical patients and it may be reasonable to prescribe them for hospitalized obese patients with COVID-19.38,47
There is also no evidence of factors associated with higher thrombotic risk to discriminate patients who would benefit from a different thromboprophylaxis approach. Furthermore, autopsy studies demonstrated the predominance of microvascular thrombosis in the lungs, coincident with markers of inflammation, which is a hallmark of prolonged infection and sepsis.48,49 As mentioned above, anticoagulant treatment for sepsis has failed in multiple studies in the past.21,25–27
Therefore, until further evidence of the benefit of increasing prophylactic doses emerges, we suggest the use of LMWH at standard dose for thromboprophylaxis, adjusted for body weight and renal function, in patients admitted to general wards or the ICU, unless there are specific contraindications. Table 2 contains the dosing regimens for VTE prophylaxis. We are aware that the apparent increased incidence of VTE in COVID-19 has led physicians to change their practice by increasing the dose of anticoagulation for prophylaxis to intermediate or therapeutic doses of LMWH in ICU patients or in patients with high D-dimers or fibrinogen levels. However, the benefit of this approach has not yet been confirmed and well-designed trials are needed to address this question.
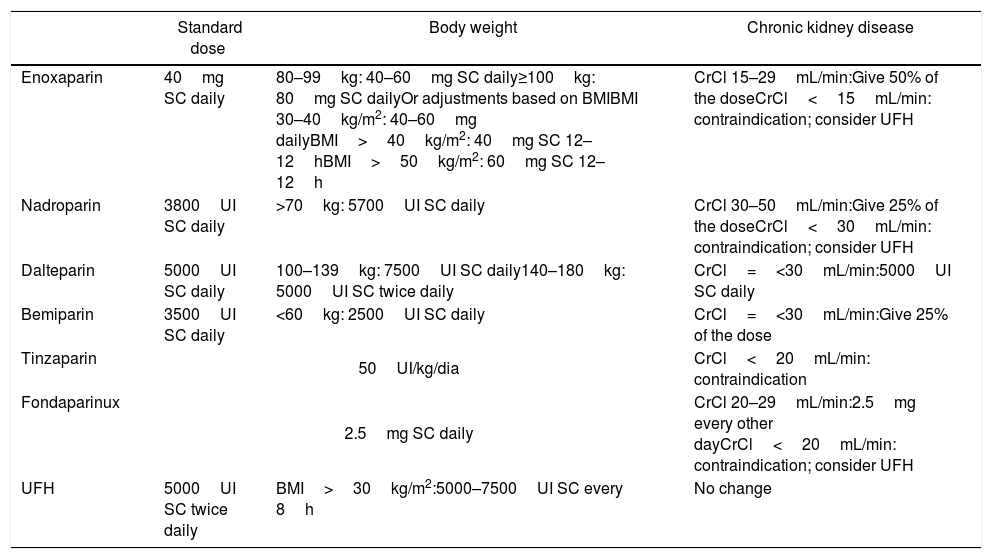
Standard dose of LMWH, UFH and fondaparinux for prophylaxis and adjustments, according to body weight and renal function.
| Standard dose | Body weight | Chronic kidney disease | |
|---|---|---|---|
| Enoxaparin | 40mg SC daily | 80–99kg: 40–60mg SC daily≥100kg: 80mg SC dailyOr adjustments based on BMIBMI 30–40kg/m2: 40–60mg dailyBMI>40kg/m2: 40mg SC 12–12hBMI>50kg/m2: 60mg SC 12–12h | CrCl 15–29mL/min:Give 50% of the doseCrCl<15mL/min: contraindication; consider UFH |
| Nadroparin | 3800UI SC daily | >70kg: 5700UI SC daily | CrCl 30–50mL/min:Give 25% of the doseCrCl<30mL/min: contraindication; consider UFH |
| Dalteparin | 5000UI SC daily | 100–139kg: 7500UI SC daily140–180kg: 5000UI SC twice daily | CrCl=<30mL/min:5000UI SC daily |
| Bemiparin | 3500UI SC daily | <60kg: 2500UI SC daily | CrCl=<30mL/min:Give 25% of the dose |
| Tinzaparin | 50UI/kg/dia | CrCl<20mL/min: contraindication | |
| Fondaparinux | 2.5mg SC daily | CrCl 20–29mL/min:2.5mg every other dayCrCl<20mL/min: contraindication; consider UFH | |
| UFH | 5000UI SC twice daily | BMI>30kg/m2:5000–7500UI SC every 8h | No change |
LMWH: low molecular weight heparin; UFH: unfractionated heparin; BMI: body mass index; SC: subcutaneous; CrCl: creatinine clearance; IU: international unit.
Prior trials have demonstrated that thromboprophylaxis should be given to medically ill patients at high risk for thrombosis during the entire hospitalization period and for at least 6–14 days.50,51 Extended duration of pharmacologic thromboprophylaxis may benefit patients with persistent immobility after discharge or those with reduced mobility and additional risk factors for thrombosis, such as older age (above 75 years), previous history of VTE, known thrombophilia, active cancer, obesity, use of estrogen, or chronic heart or respiratory failure.52
Although no specific studies on the duration of thromboprophylaxis in COVID-19 are available to date, thromboprophylaxis should be prescribed during the entire hospitalization period, unless there is a contraindication. Once there is a widespread pressure for early discharge of patients, another important consideration is to complete a minimum course of pharmacological thromboprophylaxis for at least 7 days.40
Following this rationale, we suggest maintaining thromboprophylaxis after hospital discharge for COVID-19 patients who are at high risk for VTE or maintain immobility, unless there are specific contraindications. The risk and benefits of this approach should be reevaluated periodically.
Diagnosis and treatment of venous thromboembolismDVT is suspected in case an edema, pain, redness or unilateral cyanosis of the limb is present. Suspicion of PE is based on clinical features, such as unexplained abrupt worsening in PaO2/FiO2 and hemodynamic instability, or surrogate markers, such as pulmonary hypertension, abrupt right ventricular dilatation and hypokinesis or right heart thrombus in transit on echocardiography, increase in troponin or B-type natriuretic levels.53 Acute DVT and PE should be confirmed by imaging tests, ideally being ultrasound of the lower extremities and CT pulmonary angiography, respectively.
In the case an imaging test is not feasible due to difficulties in mobilizing or positioning the patient on mechanical ventilation for CT scans, the need to protect staff from exposure to COVID-19 patients or contraindications for the test, a presumptive diagnosis of acute VTE may be performed based on clinical history, combined with physical examination and laboratory or other available tests. Given that COVID-19 patients present with a significant elevation of D-dimers at baseline6 and there is no validated cut-off value to discriminate patients at high risk for VTE,7 the diagnosis of VTE should not be based solely on the values of D-dimers.
The treatment approach for acute thrombosis must be based on current guidelines.54,55 We recommend the use of LMWH for the treatment of acute VTE. Alternatively, UFH or fondaparinux can be used. Therapeutic doses of LMWH, UFH and fondaparinux are demonstrated in Table 3.
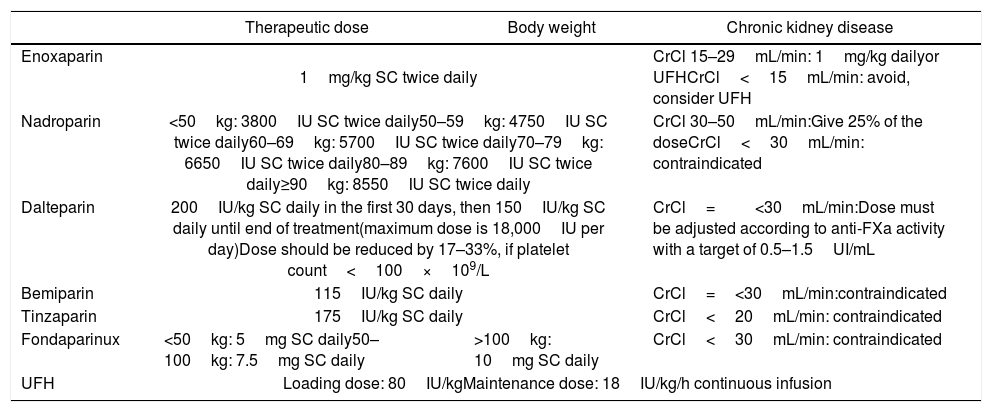
Therapeutic dose of LMWH, UFH and fondaparinux and adjustments, according to body weight and renal function.
| Therapeutic dose | Body weight | Chronic kidney disease | |
|---|---|---|---|
| Enoxaparin | 1mg/kg SC twice daily | CrCl 15–29mL/min: 1mg/kg dailyor UFHCrCl<15mL/min: avoid, consider UFH | |
| Nadroparin | <50kg: 3800IU SC twice daily50–59kg: 4750IU SC twice daily60–69kg: 5700IU SC twice daily70–79kg: 6650IU SC twice daily80–89kg: 7600IU SC twice daily≥90kg: 8550IU SC twice daily | CrCl 30–50mL/min:Give 25% of the doseCrCl<30mL/min: contraindicated | |
| Dalteparin | 200IU/kg SC daily in the first 30 days, then 150IU/kg SC daily until end of treatment(maximum dose is 18,000IU per day)Dose should be reduced by 17–33%, if platelet count<100×109/L | CrCl=<30mL/min:Dose must be adjusted according to anti-FXa activity with a target of 0.5–1.5UI/mL | |
| Bemiparin | 115IU/kg SC daily | CrCl=<30mL/min:contraindicated | |
| Tinzaparin | 175IU/kg SC daily | CrCl<20mL/min: contraindicated | |
| Fondaparinux | <50kg: 5mg SC daily50–100kg: 7.5mg SC daily | >100kg: 10mg SC daily | CrCl<30mL/min: contraindicated |
| UFH | Loading dose: 80IU/kgMaintenance dose: 18IU/kg/h continuous infusion | ||
LMWH: low molecular weight heparin; UFH: unfractionated heparin; BMI: body mass index; SC: subcutaneous; CrCl: creatinine clearance; IU: international unit.
In-hospital patients with severe COVID-19 are frequently treated with multiple drugs, including antiviral therapy which can strongly interact with DOACs.56 Given that DOACs and AVK interact with several drugs, it is highly recommended that doctors check for drug interaction before the prescription of any drug concomitantly with DOAC and AVK. In a cohort of severe COVID-19 inpatients taking DOACs and antiviral agents, Testa el al.57 have shown that DOAC plasma levels were 6 times higher during hospitalization, in comparison to the pre-hospitalization period.
Therefore, we consider reasonable that clinically stable patients admitted to the ward using DOAC or AVK continue their anticoagulant treatment during ward hospitalization if there is no relevant drug–drug interaction between the anticoagulant and the medications used to treat COVID-19. We recommend switching DOAC and AVK to LMWH if the patient is admitted to the ICU or is at risk for a significant drug–drug interaction.
Summary of the recommendationsThe analysis of the peripheral blood smear, platelet count, PT, aPTT, fibrinogen and D-dimer levels are recommended for all hospitalized patients at admission. These parameters should be regularly monitored in critically ill patients. The most adequate time interval between tests is uncertain and testing should be based on clinical indication, assays availability and the local laboratory capacity and facility.
We suggest performing venous compression Duplex scan at admission of ICU patients and then on a regular basis, whenever available and at convenient intervals, to detect DVT and to prevent its complications. We also suggest maintaining vigilance for clinical and echocardiographic signs of PE.
The diagnosis of DIC does not indicate anticoagulation, unless a thrombotic event is present. The use of anticoagulants in COVID-19 should be restricted to prophylaxis of VTE or the treatment of thrombotic events.
All patients hospitalized for suspected or confirmed COVID-19 should receive pharmacologic thromboprophylaxis, unless there are contraindications (Table 1). We suggest the use of LMWH. Alternatively, UFH or fondaparinux can be given for thromboprophylaxis. In the case that pharmacologic thromboprophylaxis is contraindicated, mechanical prophylaxis should be used.
We suggest the use of LMWH at a standard dose for thromboprophylaxis, adjusted for body weight and renal function (Table 2), for patients admitted to general wards or the ICU, unless there are specific contraindications (Table 1). We are aware that the apparent increased incidence of VTE in COVID-19 has led physicians to change their practice by increasing the dose of prophylactic anticoagulation to intermediate or therapeutic doses of LMWH in ICU patients or in patients with high D-dimers or fibrinogen levels. However, the benefit of this approach has not yet been confirmed and trials are needed to address this question.
Prophylaxis for thrombosis should be prescribed during the entire hospitalization period. It is reasonable to maintain prophylaxis after the hospital discharge for patients at high risk of thrombosis or for those with immobility, unless there are specific contraindications. The risks and benefits of this approach should be reevaluated periodically.
Acute VTE events should be confirmed by imaging tests. In the case this is not feasible, presumptive diagnosis of an acute VTE event may be made based on clinical history, combined with physical examination, laboratory and other available tests. The diagnosis of VTE should not be based solely on the values of D-dimers.
We recommend the use of LMWH for the treatment of acute VTE. Alternatively, UFH or fondaparinux can be used (Table 3).
We recommend switching DOAC and AVK to LMWH if the patient is admitted to the ICU or is at risk of significant drug–drug interaction.
Conflicts of interest:FOS is on speaker's bureau for Bayer.
MCC is a consultant/advisor for Sanofi-Aventis on VTE prophylaxis.
AVSM is a consultant/advisor for Pfizer, Bayer, Novartis, Daiichi-Sankyo, Zodiac and Roche.
ATR is on speaker's bureau for Bayer, Boehringer and Pfizer. ATR is a consultant/advisor for Pfizer and Sanofi.
ER is on speaker's bureau for Bayer, BMS/PFE, Aspen, BI, Daiichi Sankyo. ER is a consultant/advisor for Pfizer, BMS, Bayer, Sanofi, Amgen, Daiichi-Sankyo, Cristalia and Aspen.
JAB is on speaker's bureau for Pfizer, Bayer, Daiichi-Sankyo and Sanofi.
DML is on speaker's bureau for Bayer, Stago, Daiichi Sankyo, Novartis and Sanofi.
CCF is on speaker's bureau for Bayer.
The remaining authors declare no conflict of interest.