Relapsed and refractory (R/R) acute leukemia represents a challenge in clinical practice. Acute myeloid leukemia (AML) patients undergoing cytarabine-based salvage therapy achieve complete remission in 4 to 65% of cases.1 Pre-transplant complete remission status and, more recently, measurable residual disease (MRD) have also been found to be important prognostic factors in R/R-AML patients who receive hematopoietic cell transplantation (HCT). MRD-guided therapy has been the mainstay for both childhood and adult acute lymphoblastic leukemia (ALL) longer than in AML, and absence MRD is a robust prognostic factor in patients with ALL undergoing HCT. Therefore, salvage therapy strategies that can efficaciously improve disease control in acute leukemia patients prior to HCT are urgently needed.
Gemtuzumab ozogamicin (GO) is a conjugated-antibody that targets CD33, widely expressed on AML blasts and its precursors and present in about of 15% of pediatric ALL cases.2 Different studies have reported efficacy of GO in newly diagnosed and R/R-AML and CD33+ ALL, either in monotherapy or associated with other drugs.3–5 Despite being voluntarily withdrawn in 2010 from market after a large phase 3 trial with single-dosing regimen showed clinical futility and increased induction-related mortality, GO in a fractioned dosing regimen was reapproved by the U.S. Food and Drug Administration (FDA) for newly diagnosed and relapsed AML in 2017 based on the multicenter French trial Acute Leukemia French Association 701 (ALFA 701) and a large meta-analysis showing efficacy and safety.6 More recently, the European Medicines Agency (EMA) has also approved its use.7
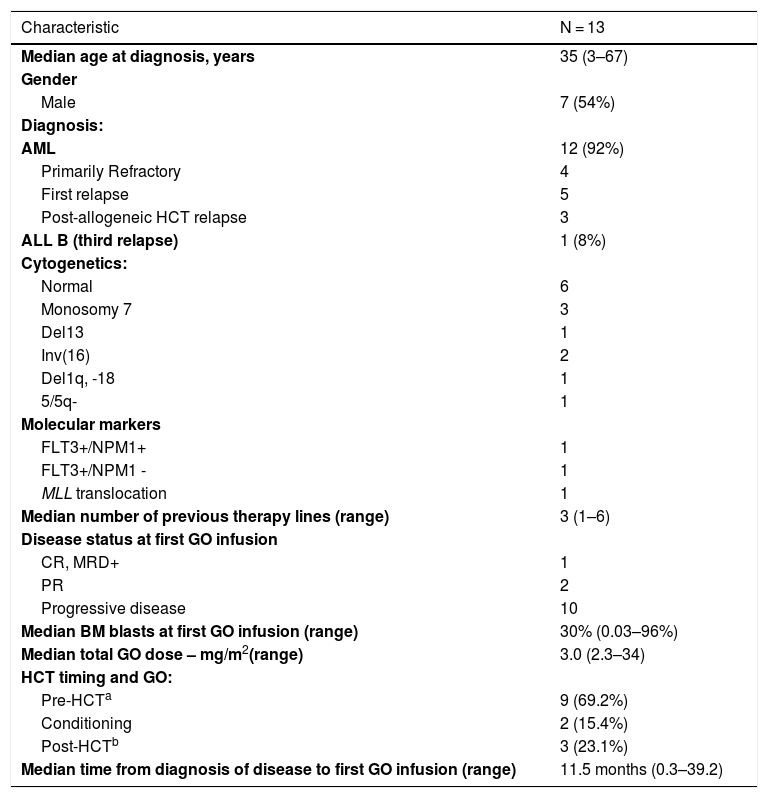
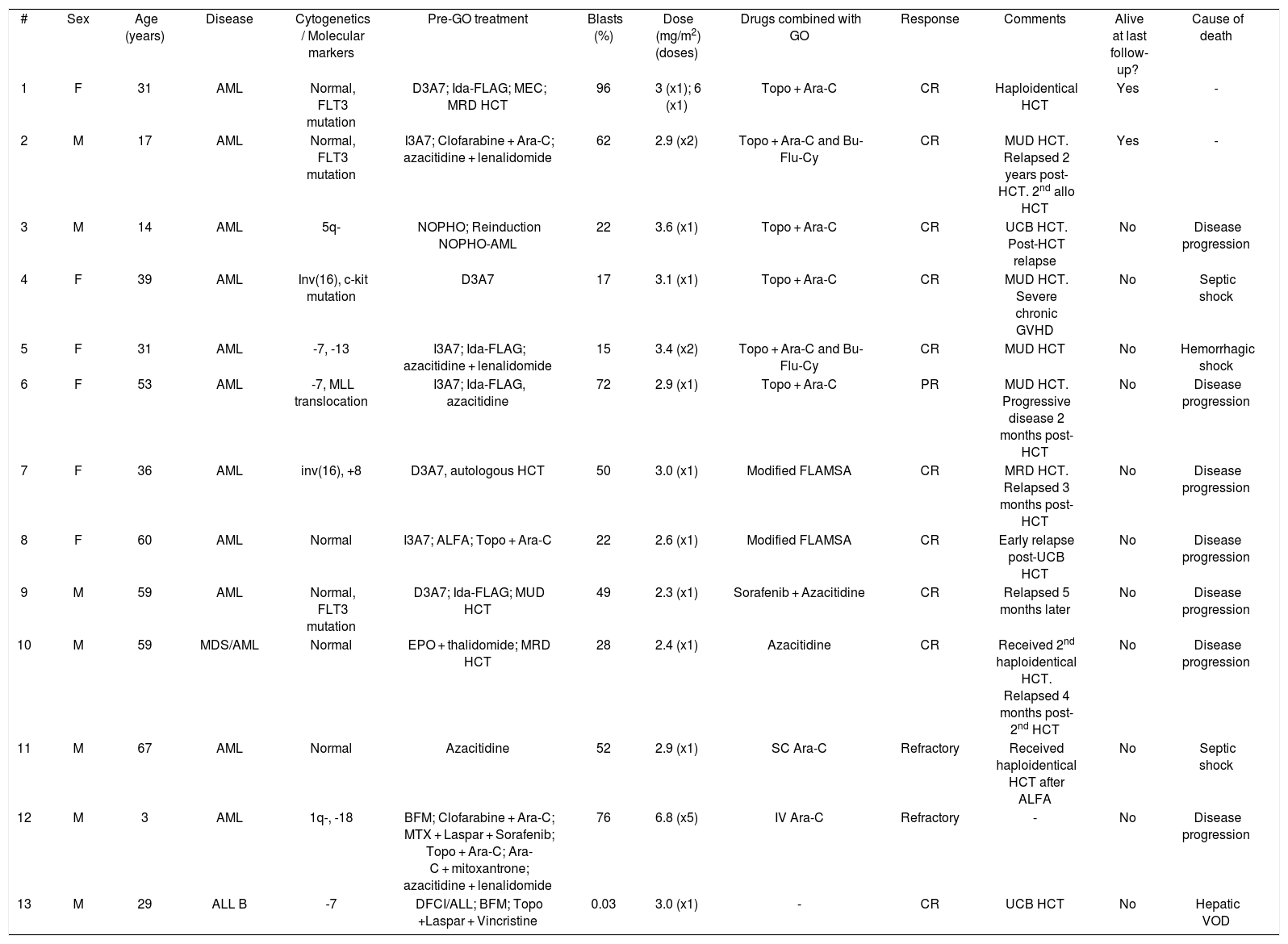
Herein, we report 13 acute leukemia cases receiving GO as compassionate drug use at any point of their treatment at Hospital Sírio-Libanês Hospital (Brazil) from 2011 to 2016. GO has not been approved in Latin America yet. Baseline characteristics are summarized in Table 1. All patients had R/R acute leukemia, most of them after several lines of therapy. GO was given in addition to multidrug chemotherapy in 12 cases (Table 2), and only one patient received it as monodrug. Four patients received GO along with their conditioning regimen prior to HCT. Out of 12 cases having active disease at the time of first GO infusion, ten achieved complete remission (CR) and one partial remission (PR). All patients, except for one, achieving CR or PR subsequently underwent HCT. A patient with relapsed CD33+ ALL in MRD-positive CR achieved MRD negativity after GO monotherapy. Median disease-free survival was 4.5 months after first GO infusion. Eleven patients eventually died: seven due to disease progression/relapse and four due to treatment-related toxicity, one of whom with hepatic veno-occlusive disease. Median overall survival, defined from first GO infusion to death, was 6.9 months. Two patients were alive at last follow-up. Since 2017, GO has been requested for two additional patients with R/R AML, but unfortunately the delay associated with international shipping and lengthy paperwork processing by the pharmaceutical company and the National Sanitary Surveillance Agency (ANVISA) prevented its timely administration.
Patient baseline characteristics.
| Characteristic | N = 13 |
|---|---|
| Median age at diagnosis, years | 35 (3–67) |
| Gender | |
| Male | 7 (54%) |
| Diagnosis: | |
| AML | 12 (92%) |
| Primarily Refractory | 4 |
| First relapse | 5 |
| Post-allogeneic HCT relapse | 3 |
| ALL B (third relapse) | 1 (8%) |
| Cytogenetics: | |
| Normal | 6 |
| Monosomy 7 | 3 |
| Del13 | 1 |
| Inv(16) | 2 |
| Del1q, -18 | 1 |
| 5/5q- | 1 |
| Molecular markers | |
| FLT3+/NPM1+ | 1 |
| FLT3+/NPM1 - | 1 |
| MLL translocation | 1 |
| Median number of previous therapy lines (range) | 3 (1–6) |
| Disease status at first GO infusion | |
| CR, MRD+ | 1 |
| PR | 2 |
| Progressive disease | 10 |
| Median BM blasts at first GO infusion (range) | 30% (0.03–96%) |
| Median total GO dose – mg/m2(range) | 3.0 (2.3–34) |
| HCT timing and GO: | |
| Pre-HCTa | 9 (69.2%) |
| Conditioning | 2 (15.4%) |
| Post-HCTb | 3 (23.1%) |
| Median time from diagnosis of disease to first GO infusion (range) | 11.5 months (0.3–39.2) |
Abbreviations: HCT stem cell transplant, AML: acute myeloid leukemia; ALL: acute lymphoid leukemia; CR: complete remission; PR: partial remission; MRD: minimal measurable disease.
Individual data.
| # | Sex | Age (years) | Disease | Cytogenetics / Molecular markers | Pre-GO treatment | Blasts (%) | Dose (mg/m2) (doses) | Drugs combined with GO | Response | Comments | Alive at last follow-up? | Cause of death |
| 1 | F | 31 | AML | Normal, FLT3 mutation | D3A7; Ida-FLAG; MEC; MRD HCT | 96 | 3 (x1); 6 (x1) | Topo + Ara-C | CR | Haploidentical HCT | Yes | - |
| 2 | M | 17 | AML | Normal, FLT3 mutation | I3A7; Clofarabine + Ara-C; azacitidine + lenalidomide | 62 | 2.9 (x2) | Topo + Ara-C and Bu-Flu-Cy | CR | MUD HCT. Relapsed 2 years post-HCT. 2nd allo HCT | Yes | - |
| 3 | M | 14 | AML | 5q- | NOPHO; Reinduction NOPHO-AML | 22 | 3.6 (x1) | Topo + Ara-C | CR | UCB HCT. Post-HCT relapse | No | Disease progression |
| 4 | F | 39 | AML | Inv(16), c-kit mutation | D3A7 | 17 | 3.1 (x1) | Topo + Ara-C | CR | MUD HCT. Severe chronic GVHD | No | Septic shock |
| 5 | F | 31 | AML | -7, -13 | I3A7; Ida-FLAG; azacitidine + lenalidomide | 15 | 3.4 (x2) | Topo + Ara-C and Bu-Flu-Cy | CR | MUD HCT | No | Hemorrhagic shock |
| 6 | F | 53 | AML | -7, MLL translocation | I3A7; Ida-FLAG, azacitidine | 72 | 2.9 (x1) | Topo + Ara-C | PR | MUD HCT. Progressive disease 2 months post-HCT | No | Disease progression |
| 7 | F | 36 | AML | inv(16), +8 | D3A7, autologous HCT | 50 | 3.0 (x1) | Modified FLAMSA | CR | MRD HCT. Relapsed 3 months post-HCT | No | Disease progression |
| 8 | F | 60 | AML | Normal | I3A7; ALFA; Topo + Ara-C | 22 | 2.6 (x1) | Modified FLAMSA | CR | Early relapse post-UCB HCT | No | Disease progression |
| 9 | M | 59 | AML | Normal, FLT3 mutation | D3A7; Ida-FLAG; MUD HCT | 49 | 2.3 (x1) | Sorafenib + Azacitidine | CR | Relapsed 5 months later | No | Disease progression |
| 10 | M | 59 | MDS/AML | Normal | EPO + thalidomide; MRD HCT | 28 | 2.4 (x1) | Azacitidine | CR | Received 2nd haploidentical HCT. Relapsed 4 months post-2nd HCT | No | Disease progression |
| 11 | M | 67 | AML | Normal | Azacitidine | 52 | 2.9 (x1) | SC Ara-C | Refractory | Received haploidentical HCT after ALFA | No | Septic shock |
| 12 | M | 3 | AML | 1q-, -18 | BFM; Clofarabine + Ara-C; MTX + Laspar + Sorafenib; Topo + Ara-C; Ara-C + mitoxantrone; azacitidine + lenalidomide | 76 | 6.8 (x5) | IV Ara-C | Refractory | - | No | Disease progression |
| 13 | M | 29 | ALL B | -7 | DFCI/ALL; BFM; Topo +Laspar + Vincristine | 0.03 | 3.0 (x1) | - | CR | UCB HCT | No | Hepatic VOD |
Abbreviations: AML: acute myeloid leukemia; D3A7: daunorubicin x 3 + cytarabine x 7; Ida-FLAG: idarubicin + fludarabine + cytarabine + granulocyte colony stimulating factor; MEC: mitoxantrone + etoposide + cytarabine; MRD: matched related donor; HCT: hematopoietic cell transplant; Topo: topotecan; Ara-C: cytarabine; CR: complete remission; I3A7: idarubicin x 3 + cytarabine x 7; Bu-Flu-Cy: conditioning regimen based on busulfan + fludarabine + cyclophosphamide; MUD: matched unrelated donor; allo: allogeneic; NOPHO: pediatric AML protocol by the Nordic Society of Pediatric Hematology and Oncology; UCB: unrelated cord blood; GVHD: graft-versus-host disease; PR: partial remission; Modified FLAMSA: fludarabine + mitoxantrone + cytarabine; ALFA: Acute Leukemia French Association 9803 elderly protocol; EPO: erythropoietin; SC: subcutaneous; BFM: pediatric AML protocol by the Berlin-Frankfurt-Munster Group; MTX: methotrexate; Laspar: L-asparaginase; IV: intravenous; ALL: acute lymphoid leukemia; DFCI/ALL: protocol by the Dana-Farber Cancer Institute/ALL Consortium; VOD: veno-occlusive disease.
The interpretation of this retrospective case series is limited given its small number, multiple salvage therapies and differences in GO dosing and timing. Although not directly comparable to previous studies, the high proportion (10 out of 12) of patients with active R/R AML achieving complete remission following GO-based therapy is remarkable. Among the six patients receiving topotecan and cytarabine, the most common drug combination with GO in our cohort, all except one achieved CR, in contrast to a previous study that showed a CR proportion of only 12% with the same combination in R/R AML.8 However, these patients received a higher dose of GO (at least 9 mg/m2) compared to the present study (median of 3 mg/m2 in the topotecan + cytarabine subset), and the fact that 29% of them had early death due to toxicity, mainly severe transaminitis and/or VOD, may explain at least in part the lower response reported by the authors.8 GO combinations with other drugs have been reported to yield CR in 12–88% of R/R AML patients,9 yet these studies have significant heterogeneity in treatment, patient and disease-related characteristics.
The use of GO in our cohort was overall safe. Infusion reactions were negligible (data not shown). Hepatic veno-occlusive disease (VOD), the toxicity of greatest concern, only occurred in one ALL patient shortly after a myeloablative HCT, yet he had received only a single dose of GO at 3 mg/m2 and had multiple additional risk factors for hepatic VOD. The incidence of hepatic VOD has been reported to be low when individual doses were equal to or lower than 3 mg/m2,10 which prompted FDA reapprove GO in fractionated low-dosing regimens. In this case series, most patients received only one dose of GO and at doses not greater than 3 mg/m2, which might explain the low incidence of VOD considering that GO was administered shortly prior to or during HCT for most patients.
In summary, GO at low doses seemed to be efficacious and relatively safe as a bridge to allogeneic HCT in this case series of R/R acute leukemia patients, yet these results need to be taken cautiously due to the small number and heterogeneity of this report. Despite the high proportion of CR, PFS was brief with most patients relapsing shortly after HCT. Although GO has been approved by FDA only as monotherapy in R/R AML, prospective randomized trials combining different regimens of fractionated GO with multidrug chemotherapy and new agents (e.g., venetoclax) might deepen disease control yielding a high rate of CR and improved PFS in the future.
Author contributionsG.F. wrote the manuscript, G.F. and G.R.S. collected data from medical charts. All authors revised and approved the final manuscript.
Conflicts of interestThe authors declare no conflict of interest.