CD28 null T helper (Th) cells are rare in healthy individuals, but they are increased in various inflammatory and immune-mediated diseases. In this study, we determined the size of the CD4+/CD28 null T lymphocyte compartment in the peripheral blood of 40 autoimmune hemolytic anemia (AIHA) patients (idiopathic and secondary) and 20 healthy control subjects, using tri-color flow cytometry. The frequency and absolute count of CD4+/CD28 null T helper (Th) cells was significantly higher in idiopathic AIHA patients, compared to healthy controls (p = 0.001 and 0.001, respectively) and to patients with secondary AIHA (p = 0.04 and 0.01, respectively). The percentage of CD4+/CD28 null Th cells was also negatively correlated to the hemoglobin (Hb) level (p = 0.03). These findings demonstrate, for the first time, the expansion of this phenotypically-defined population of T lymphocytes in patients with idiopathic AIHA and indicate that it likely plays an etiological role in the development of this disease. However, establishing the use of this marker for diagnosis or monitoring treatment of such patients needs further studies.
Autoimmune diseases represent a significant clinical entity. They represent a state of self-tolerance loss that leads to the production of self-reacting antibodies. One of the well-known autoimmune diseases is the autoimmune hemolytic anemia (AIHA), in which the survival of self-red blood corpuscles is shortened due to their destruction by circulating autoantibodies. Although this disease has been well-recognized for over 150 years, AIHA still represents a diagnostic, prognostic, and therapeutic challenge. The only hope for the cure of such a disease is to restore the immune tolerance back to normal.
In parallel, an essential component of our adaptive immune system is the T lymphocyte. After their maturation in the thymus, normal T lymphocytes circulate in the blood until they recognize their particular antigen on the surface of the antigen-presenting cells (APCs). This is the first step in their activation, while the second signal is provided by the interaction of certain T-cell surface receptors with their specific ligands on the APC surface. Those surface receptors on the T cell surface are known as costimulatory pathways.
One of the most studied costimulatory pathways is the pathway provided by the interaction of CD28 (on the T-cell surface) with its APCs surface ligands B7-1 (CD80) and B7-2 (CD86).
The CD4+/CD28 null T lymphocytes were significantly increased in many autoimmune and inflammatory diseases, but, to our knowledge, no previous studies investigated the presence or significance of CD 28 null T lymphocytes in patients with autoimmune hemolytic anemia. Consequently, we aimed to study this lymphocyte population with the CD4+/CD28 null Th effector phenotype among patients with autoimmune hemolytic anemia and to correlate its presence with the hemoglobin level in such patients.
Study designSubjectsThe study included sixty subjects. Forty patients with newly diagnosed AIHA were enrolled in the study. Twenty patients had primary or idiopathic AIHA (group 1) and 20 patients had AIHA secondary to autoimmune diseases, namely systemic lupus erythematosus (SLE) in 17 patients and rheumatoid arthritis in 3 patients, (group 2). Twenty healthy age- and sex-matched subjects were included as a control group (group 3).
The exclusion criteria at the time of sampling included current steroid therapy, recent blood transfusions, active Hepatitis C virus infection and other concurrent chronic inflammatory conditions.
- ‐
Patients underwent clinical follow-up throughout the study duration (48 months), with a median follow-up time of 32 months.
- ‐
All procedures performed in our study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
- ‐
Informed consent was obtained from all the participants in the study.
The following was done for all patients:
All patients were diagnosed with autoimmune hemolytic anemia via detailed history-taking, clinical examination and routine laboratory investigations, including complete bloodwork with differential leukocyte count, reticulocyte count, erythrocyte sedimentation rate (ESR), serum lactate dehydrogenase (LDH), direct Coombs test, liver function tests (including bilirubin levels), kidney function tests (including uric acid), hepatitis markers (HCV Ab/PCR; HBVsAg/PCR and HIV Ab) and collagen markers (including ANA titer, anti-ds DNA, ASMA, rheumatoid factor, AntiCPP, and ANCA).
- ‐
Bone marrow aspiration and trephine biopsy were performed for all patients to exclude bone marrow infiltration by malignancies or granuloma (especially leukemia or lymphoma as secondary causes of AIHA).
Peripheral blood samples from patients and controls were analyzed within 24 h from collection. Cells were labeled by incubating 106 nucleated cells with saturating amounts of fluorochrome-conjugated mouse anti-human antibodies for 20 min at room temperature in the dark. The antibodies were set in a combination of CD3 PC5/CD 4 FITC/ CD28 PE (BD Pharmingen, BD biosciences, Heidelberg, Germany). For erythrocyte lysis, cells were incubated for 2 min with 1 ml 0.83% ammonium chloride and then centrifuged for 5 min at 500 × g cells. Then cells were re-suspended in a mixture of phosphate buffer saline (PBS) pH 7.2 and 1% paraformaldehyde fixative. Murine isotype matched antibodies (Ig G2a) were used as an isotype control for each sample.
Data from 10,000 to 15,000 events per sample were acquired, analyzed and processed using the SYSTEM II software, Version 3, Coulter electronics on Epics XL FCM (Coulter Electronics, USA). The gating of cells was initially set with forward scatter (FS) and side scatter (SC), followed by the gating of CD3+ cells (T-cells). The CD3+CD4+ cells (T-helper) expressing or lacking CD28 expression were quantified both as a percentage and an absolute count. Each population was identified as a compartment of the terminal effector phenotype.12
Statistical analysisStatistical analyses were performed using the SPSS software version 13.0. The results were non-parametric and were thus expressed as median and range. Comparisons between groups were performed using the Mann Whitney U test and intra-group relations between parameters were analyzed using the Spearman correlation coefficient. A probability of <0.05 indicated statistical significance.
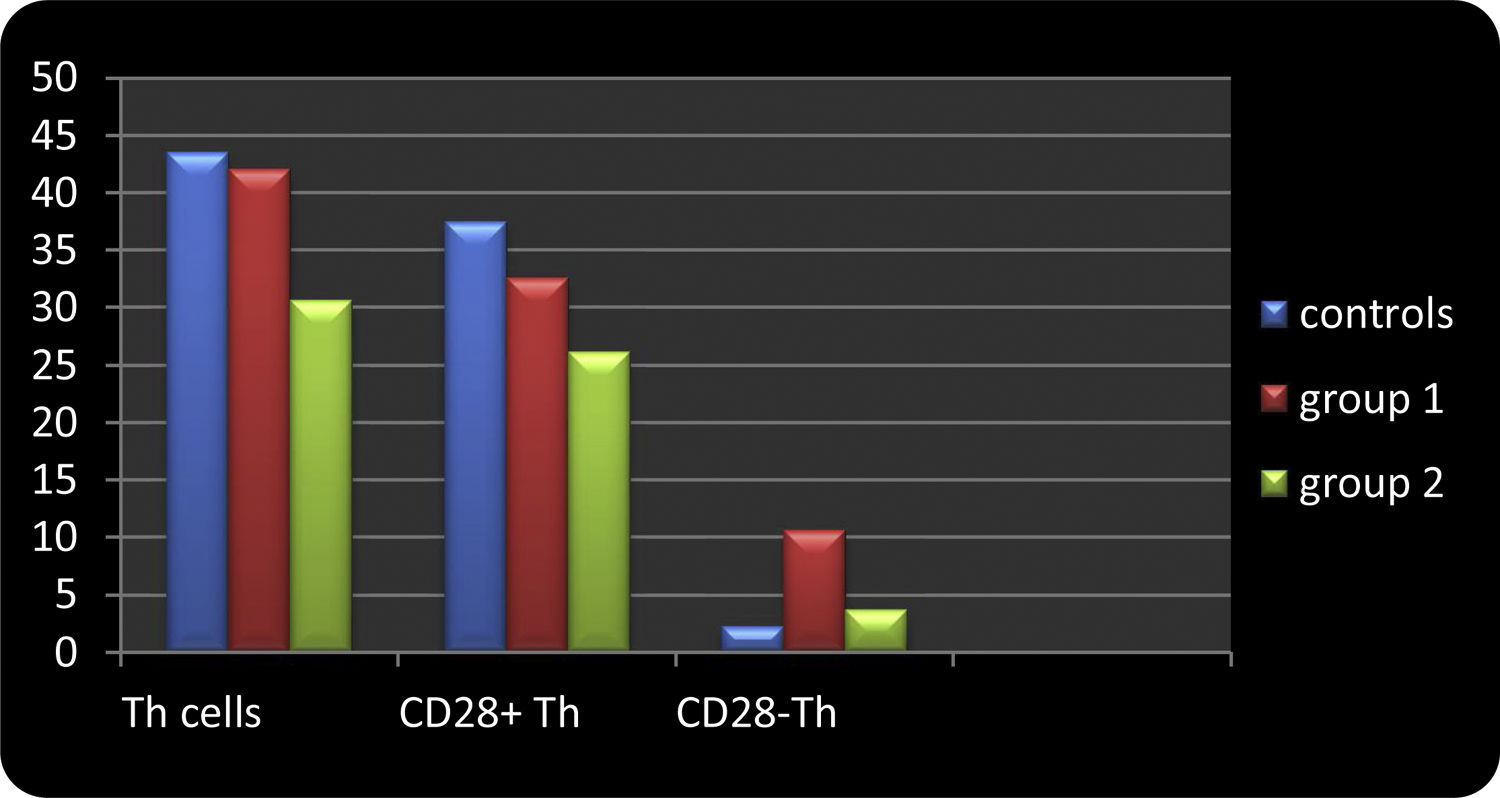
ResultsThe data is presented in Table 1 and Figure 1.
Frequency of lymphocytes and their T helper phenotype subsets, according to CD28 expression in AIHA patients, compared to controls.
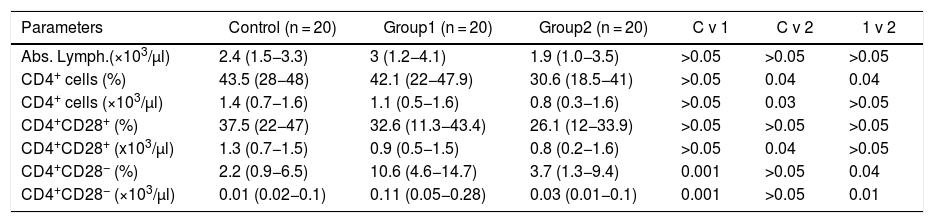
| Parameters | Control (n = 20) | Group1 (n = 20) | Group2 (n = 20) | C v 1 | C v 2 | 1 v 2 |
|---|---|---|---|---|---|---|
| Abs. Lymph.(×103/μl) | 2.4 (1.5−3.3) | 3 (1.2−4.1) | 1.9 (1.0−3.5) | >0.05 | >0.05 | >0.05 |
| CD4+ cells (%) | 43.5 (28−48) | 42.1 (22−47.9) | 30.6 (18.5−41) | >0.05 | 0.04 | 0.04 |
| CD4+ cells (×103/μl) | 1.4 (0.7−1.6) | 1.1 (0.5−1.6) | 0.8 (0.3−1.6) | >0.05 | 0.03 | >0.05 |
| CD4+CD28+ (%) | 37.5 (22−47) | 32.6 (11.3−43.4) | 26.1 (12−33.9) | >0.05 | >0.05 | >0.05 |
| CD4+CD28+ (x103/μl) | 1.3 (0.7−1.5) | 0.9 (0.5−1.5) | 0.8 (0.2−1.6) | >0.05 | 0.04 | >0.05 |
| CD4+CD28− (%) | 2.2 (0.9−6.5) | 10.6 (4.6−14.7) | 3.7 (1.3−9.4) | 0.001 | >0.05 | 0.04 |
| CD4+CD28− (×103/μl) | 0.01 (0.02−0.1) | 0.11 (0.05−0.28) | 0.03 (0.01−0.1) | 0.001 | >0.05 | 0.01 |
C v 1: comparison of controls to group 1; C v 2: comparison of control to group 2; C v 3: comparison of group 1 to group 2.
Comparison between controls and patient groups in relation to compartments of T helper effector populations. This figure shows that the percentage of CD4+CD28 null T cells was significantly higher among group 1 (idiopathic AIHA) patients (median percentage of 10.6%), compared to controls (median percentage of 2.2%) (p = 0.001 and 0.001, respectively) and to group 2 (secondary AIHA) patients (median percentage of 3.7%) (p = 0.04 and 0.01, respectively). The percentage of CD4+CD28− T cells in group 2 patients were comparable to the control group (p > 0.05 and p > 0.05, respectively).
Clinical characteristics of patients (groups 1 and 2)
The study included 60 subjects (40 with AIHA and 20 matching healthy controls). Among the 40 AIHA patients, there were 10 males and 30 females, whose ages ranged from 18 to 59 years, with a mean of 38 ± 19 years. Patients were subdivided into two groups:
Group 1 was comprised of 20 patients having primary AIHA. Nine of the 20 (45%) suffered from severe anemia (Hb < 6 g/dl), while 11/20 (55%) had Hb > 6 g/dl.
Group 2 was comprised of 20 patients having secondary AIHA, of which 17/20 (85%) were cases of AIHA secondary to systemic lupus erythematosus and 3/20 (15%) patients had AIHA secondary to rheumatoid arthritis. Two of the 20 patients (10%) suffered from severe anemia (Hb < 6 g/dl), while 18/20 (90%) had Hb > 6 g/dl.
Severe anemia (Hb < 6.0 gm/dl) was significantly more frequent among group 1 patients (9/20, 45%), compared to group 2 patients (2/20, 10%) (p = 0.03).
No significant difference was found between group 1 and group 2 patients, regarding other clinical findings.
The study also included 20 healthy subjects as a control group (group 3). It was comprised of 8 males and 12 were females, with ages ranging from 22 to 57 years, constituting a mean of 40 ± 18 years. The total and absolute lymphocyte counts were comparable in patients and controls (p > 0.05) (Table 1).
Flow cytometric analysis of lymphocytes and their T helper effector phenotype
The percentage and absolute count of CD4+ cells (T helper cells) were significantly lower in group 2 (median percentage = 30.6% and count = 0.8 × 103 cells/μl), compared to controls (median percentage = 43.5% and count = 1.4 × 103 cells/μl) (p = 0.04 and 0.03, respectively). The percentage of CD4+ cells, and not their absolute count, was significantly lower in group 2, compared to group 1 patients (median percentage = 42.1%) (p = 0.04). Group 1 patients were comparable to controls, regarding the percentage and absolute count of CD4+ cells (p > 0.05 and p > 0.05, respectively) (Table 1 and Figure 1).
The percentage and absolute count of CD4+CD28+ T cells were not significantly different in group 1 patients, compared to controls, nor was it different in group 1, compared to group 2 (p > 0.05 and p > 0.05, respectively). However, group 2 patients had significantly lower absolute CD4+CD28+ cells than the control group (p = 0.04) (Table 1 and Figure 1).
No significant correlation was established between the size of this T cell compartment and any of the other parameters studied in group 1 or group 2 patients.
The percentage and absolute count of CD4+CD28 T cells were significantly higher among group 1 patients (median percentage = 10.6% and count = 0.11 × 103 cells/μl), compared to controls (median percentage = 2.2% and count = 0.01 × 103 cells/μl) (p = 0.001 and 0.001) and to group 2 patients (median percentage = 3.7% and count = 0.03 ×103 cells/μl) (p = 0.04 and p = 0.01, respectively). The percentage and absolute count of CD4+CD28− T cells in group 2 patients were comparable to the control group (p > 0.05 and p > 0.05, respectively) (Table 1 and Figure 1).
The percentage of CD4+CD28− T cells was negatively correlated to the hemoglobin level among group 1 patients, but not among group 2 patients (r = −0.68, p = 0.03).
There was no significant correlation between the percentage of these cells and the ESR, reticulocyte count or titer of Coombs test in either group 1or 2 patients, nor was there a correlation between percentages of CD4+CD28 null T cells and the positivity of collagen markers or serum creatinine level in group 2 patients (p > 0.05 in all).
DiscussionLoss of self-tolerance comes in the foreground as the main cause of developing autoimmune diseases. In these diseases, the immune system turns into an enemy and starts producing self-attacking autoantibodies. It is still not fully understood why autoimmune diseases occur. One of the well-recognized autoimmune diseases is the autoimmune hemolytic anemia (AIHA), in which self-reacting autoantibodies attack the self-red blood corpuscles, leading to their shortened survival. Treatment of AIHA depends upon immune suppression via corticosteroids or other drugs, such as rituximab. Despite the established immune basis of AIHA, not all patients respond properly to immune suppressive therapy. In parallel, immune suppressive therapy may result in life-threatening complications and infections. The only hope to cure such a disease is to restore the immune tolerance back to normal. Thus, understanding the underlying immune disturbances is a crucial step.
The T lymphocytes play a central role in the development of autoimmune diseases. In normal individuals, normal T lymphocytes circulate in the blood until they recognize their particular antigen on the surface of the antigen-presenting cell (APC). This is the first step in their activation, while the second signal is provided by the interaction of certain T cell surface receptors with their specific ligands on the APC surface. Those surface receptors on the T cell surface are known as costimulatory pathways.
The CD 28 was the first and remains the most extensively studied costimulatory pathway. The CD28 on the surface of T lymphocyte interacts with its ligands, namely B7-1 (CD80) or B7-2 (CD86) on the APC surface. This represents the activating signal of the T cell proliferation and functions, while cytotoxic T lymphocyte-associated antigen 4 (CTLA4), which also binds to the same APC antigens (B7-1 and B7-2), provides an inhibitory signal.1
After its activation, the CD28 lowers the T cell receptor (TCR) activation threshold, leading to an increased cytokine production and increased cell survival. On the contrary, the CD28 may also augment the development and homeostasis of CD4+CD25+ Treg cells. Treg cells are negative regulators of T cell signaling. They contribute to T cell anergy and to the maintenance of self-tolerance. Thus, the CD28 can deliver either pro-inflammatory or anti-inflammatory signals.1
In our study, we used immunophenotyping to quantify the size of the CD28 null T helper effector cell compartment in peripheral blood of patients with AIHA, versus healthy controls. We found that the size of the CD28 null Th cell compartment was significantly increased among patients suffering from idiopathic AIHA, compared to healthy control subjects or to patients suffering from AIHA secondary to other autoimmune diseases. This is the first report of the expanded CD4+/CD28 null T cell population in humans with autoimmune hemolysis.
Moreover, in our study, the size of the CD28 null Th cell compartment (represented by both percentage and absolute count) was negatively correlated with the Hb level among group 1 patients
CD28 null T cells are long-lived directly cytotoxic CD4+T lymphocytes.5 They are highly differentiated effector CD4+ T cells that have downregulated the co-stimulatory molecule CD28, due to persistent antigenic stimulation.8 They function as pro-inflammatory cells because of their ability to produce significantly more interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α) and IL-2.2 Moreover, they show defective apoptosis.3 CD4+CD28 null T cells are resistant to apoptotic cell death, despite normal expression of death-inducing receptors, such as CD95 (Fas). This resistance to apoptosis is mediated by the up-regulation of the anti-apoptotic protein Bcl-2. Resistance to apoptosis may also explain the unusual longevity and persistence of this unique T cell subtype.29
In addition, CD4+CD28null T cells are cytotoxic and effective killers. This function is mediated by cytolytic enzymes, such as perforin, granzyme A and granzyme B, expressed by CD4+CD28null T cells. These cytolytic enzymes are usually present in cytotoxic CD8+ T lymphocytes and natural killer (NK) cells, whereas normal CD4+ T cells lack them.29
Another unique feature of CD4+CD28null T cells is the expression of the C-type lectin receptor NKG2D and a variety of NK cell-related receptors that belong to the killer immunoglobulin-like receptor (KIR) family.29
The CD 28null T cells may thus contribute to the pathogenesis of AIHA via their direct cytotoxic abilities and their resistance to apoptosis. The CD4+CD28null T cells may escape from the control of Treg cells and drive inflammation indefinitely. Furthermore, they secrete variable inflammatory cytokines, especially interferon-γ, which is known to suppress hematopoiesis.
Although all 40 patients studied have an autoimmune disease (group 1 and 2), the expanded CD4+CD28 null lymphocytes were only seen in patients with idiopathic AIHA and not those with secondary AIHA. This would suggest that autoimmunity per se is not the cause of the expanded CD4+CD28 null population, since everyone in group 2 had some form of autoimmune disease (either SLE or RA), as well. As severe anemia was more prevalent in group 1, and given that Hb levels were correlated with the CD4+CD28 null frequency, we thus think that the expanded CD4+CD28 null lymphocytes are more related to the anemic status than the autoimmunity process itself.
It is also possible that the accumulation of CD28 cells is an epiphenomenon, caused by repeated antigen exposure and associated chronic inflammatory state.4
It was previously shown that CD28 is constitutively expressed on all naive T cells in mice, as well as almost all CD4+ T cells and the majority of CD8+ T cells in humans.4 Furthermore, the percentage of CD28− CD4+ is rare in healthy individuals and increases with aging and with chronic inflammation or infection. Unlike naive T cells, memory/Ag-experienced T cells may downregulate CD28 on their surface, with diminished reliance on costimulatory signals.4
Loss of CD 28 on circulating CD4 T lymphocytes was studied in different types of diseases. For example, in the pediatric sickle cell, CD4+CD28 null T lymphocytes were significantly higher in patients than controls.5
In addition, CD4+CD28null T lymphocytes were significantly higher in patients with rheumatoid arthritis,6 children with active juvenile idiopathic arthritis,7 bronchiolitis obliterans syndrome,8 sarcoidosis,9 inflammatory bowel disease,10 Crohn’s disease,11 multiple sclerosis,12 Graves’ disease13 and chronic hepatitis B.14
CD4+/CD28− T cells were also studied in cervical cancer,15 delayed renal graft function16 and post-operative atrial fibrillation,17 and it was found to be significantly increased in each.
In DM patients, CD4+CD28 null T-cells were linked with poor glycemic control and the occurrence of a first cardiovascular event. In parallel, the rat model of the polycystic ovary syndrome showed a significantly higher number of CD28− cells.18
Moreover, CD4+/CD28 null T cells had a higher count in early atherosclerotic damage in patients with end-stage renal disease undergoing hemodialysis19 and in patients showing poor response to recombinant erythropoietin treatment 20.
Thus, the presence of CD4+/CD28 null T cells seems to be correlated with autoimmune, inflammatory and, possibly, neoplastic diseases. The accumulation of scientific evidence over decades points to a central role of the immune system in developing different types of diseases. This theory was proved by research work, laboratory investigations and clinically, by the successful results of immune suppressive therapy. However, it has been observed that suppressing immunity will not always give satisfactory results. Moreover, many patients develop serious complications due to immune compression, mainly via opportunistic infections. We think that the immune system is too valuable to be totally suppressed. We need our immunity to achieve a cure. It would be better to “modulate” immunity to play on our side and defend us, instead of suppressing the whole immune system. Modulated immunity may be the missing part that we need to achieve better treatment results among our patients. One of the promising key players in the immune system is the CD28. Modulation of central molecules like CD28 may open the way for fundamental new therapeutic modalities to help cure many diseases.
Despite the opposing functions of CD28, as previously mentioned, in rodents, low doses of CD28-specific superagonistic monoclonal antibodies selectively activate regulatory T cells (Treg). This observation was transmitted to human research. However, a convincing explanation for this phenomenon is still lacking.21
Targeting the CD28/CTLA-4 costimulatory pathway started many years ago. The first developed molecule was the recombinant CTLA-4Ig. When CTLA-4Ig binds with CD80 and CD86, it prevents access of CD28 to these ligands; consequently, inhibition of T lymphocytes occurs, leading to a reduction in pro-inflammatory cytokine production.22
Of this family, Abatacept was approved by the FDA in 2005 for the treatment of rheumatoid arthritis (RA). Clinical trials were conducted on patients with established RA refractory to methotrexate or TNF therapy (or both).23 Abatacept treatment significantly reduced the progression of structural damage in RA patients.24
In 2011, its second-generation, Belatacept, was approved by the FDA. Belatacept shows a higher-avidity binding for CD86 than Abatacept. It was approved for the prevention of the acute rejection following a kidney transplant.25
Recently, FR104, a novel monoclonal antibody fragment that antagonizes CD28, was developed. This CD28 antagonist selectively and efficiently prevents CD28 interaction with B7 molecules, while sparing the CTLA-4 co-inhibitory signal. The net effect of CD28 antagonism is downregulating effector T-cells, while promoting regulatory T cell (T-reg) activity.26
Studies on FR104 showed that it is as potent as Abatacept in reducing clinical symptoms, inflammation and Ab serum levels. It was studied in rhesus monkey27 and baboons.28 Moreover, in 2016, FR104 underwent a Phase 1 trial, demonstrating that it is safe and well-tolerated and has the potential to show clinical activity in autoimmune diseases, particularly in rheumatoid arthritis.26
Autoimmune hemolytic anemia represents a significant cause of morbidity and mortality, despite advances in therapy. The first-line therapy for warm AIHA is immunosuppression via corticosteroids. This therapy is effective in only 70–85% of patients. This is because of our limited understanding of the mechanism behind the development of autoantibodies in this disease. A better understanding of why AIHA occurs will definitely lead to better treatment options, survival and quality of life for those patients. Moreover, AIHA may complicate many other autoimmune and malignant diseases, such as chronic lymphocytic leukemia, acute lymphoblastic leukemia and systemic lupus erythematosus. The associated immune hemolysis adds to the risk of the original disease and may be the direct cause of death in many patients.
The centrality of CD28 and its possible role in many autoimmune, inflammatory and neoplastic diseases render targeting CD28 an attractive option. This may help many patients in different fields of medicine. Further experimental and human studies are still lacking to prove the best way to transmit basic science into human benefits.
Conflicts of interestThe authors declare no conflicts of interest.