Although extracorporeal photopheresis (ECP) is a promising second-line therapy in the treatment of chronic graft-versus-host disease (cGVHD), its use is limited by its high cost. This study aims to describe the clinical evolution of patients who underwent ECP therapy for cGVHD and to perform an economic analysis of the therapy
MethodsThis was a case series between 2016 and 2020 describing the clinical response to ECP and a micro-cost analysis of the therapy using time-driven activity-based costing.
ResultsSix patients underwent ECP for corticosteroid-dependent cGVHD The cost per ECP session is 14,960.90 Brazilian reais (BRL), which primarily consists of the ECP kit with an activator (82.78%), followed by the hospital's physical structure (14.66%), human resources (2.48%) and exams/inputs (0.08%). The number of sessions performed ranged from 2 to 42. The total cost of the therapy per patient ranged from BRL 30,000 to 500,000.
ConclusionThe response of the patient with cGVHD to treatment with ECP was variable. These micro-costing results can be used to develop remuneration and cost control strategies in hematopoietic stem cell transplantation programs, as well as in further economic studies.
Patients undergoing hematopoietic stem cell transplantation (HSCT) are at a high risk for immediate and late complications, with graft-versus-host disease (GVHD) being one of the most prevalent and having the highest mortality.1 Chronic GVHD (cGVHD) affects between 35% and 50% of HSCT recipients and is especially challenging due to the heterogeneity of its clinical presentation and treatment response. It can affect the skin, liver, eyes, lungs, joints, fascia, genitals, oral cavity and gastrointestinal tract.2–4
Less than 20% of patients with cGVHD have a complete or partial durable response to first-line corticosteroid treatment and survive one year after initial treatment without additional systemic therapy.5 The persistence of the symptoms and the deleterious effects of chronic corticosteroid use limit performance and impair productive capacity, with a significant impact on the patient's quality of life.6,7
Extracorporeal photopheresis (ECP), a second-line treatment option for GVHD, consists of the following steps: removal of mononuclear cells from the patient's blood through an apheresis process; application of photoactivator 8-methoxypsoralen in the selected cells; exposure to ultraviolet radiation A, and; reinfusion of this product into the patient.8,9 This procedure has a prolonged immunomodulatory effect, as it causes apoptosis of alloreactive T cells and promotes immunological tolerance. Compared to other therapies, ECP has the advantage of preserving the graft-versus-leukemia effect, as well as reducing the risk of opportunistic infections, as it has an immunomodulatory effect, rather than an immunosuppressive one.8,10–12
Regarding the response to treating cGVHD with ECP, some studies report an overall response > 50% in at least one of the sites affected by GVHD, while other studies describe a 50% improvement in the clinical manifestations of the disease.13 However, treatment response varies in specific organs, with a greater tendency toward improvement in skin and oral mucosa.14 Although ECP is a promising therapy and has a better response rate than other second-line therapies, no studies have presented consistent data on its effects, so it is still controversial.15
Moreover, its use is also limited by its high cost (equipment and procedure). In some cases, this cost can be offset by reducing immunosuppressive therapy, which directly influences outcomes such as hospitalization, quality of life and, sometimes, mortality related to immunosuppressive therapy.16 However, clinical follow-up studies and economic studies of ECP therapy are still scarce. Thus, this study aims to describe the clinical evolution of patients undergoing ECP as a second-line therapy for cGVHD and to perform an economic analysis of the treatment process.
MethodsThis case series describes the clinical response to ECP and an economic analysis of all patients who underwent the therapy at the Hospital de Clínicas de Porto Alegre (HCPA) between November 2016 and September 2020. The HCPA is a reference institution in HSCT in the state, with over 25 years of experience in transplantation, boasting a qualified multidisciplinary staff in all stages of care.
ECP protocolThe ECP was performed using the “in-line” method with the Therakos™ UVAR® XTS™ system. In this method, all three phases (leukapheresis, photoactivation and reinfusion) are performed sequentially in a cardiopulmonary bypass using a single device.8,9 The equipment cost was not included in this analysis, as our institution received the machine as a donation and the objective was to assess the session cost per patient. A central venous catheter (CVC) was implanted in the patients undergoing treatment. The protocol provided for two sessions per week on consecutive days until the lesions had improved and the corticosteroid dose had been reduced by approximately 50%. After this phase, the sessions occurred fortnightly until the complete cessation of the corticosteroid therapy was possible without GVHD symptom relapse.17
The response to ECP therapy was defined as: a) complete - disappearance of cGVHD in all organs; b) partial - at least 50% improvement; c) stable disease - less than 50% improvement, and; d) treatment failure/disease progression - no significant response to ECP, worsening GVHD in at least one organ or death after one month or more of ECP therapy.2,18 The clinical characteristics of the patients were assessed through a review of the electronic medical records, with the global grading of chronic GVHD performed according to Jagasia et al.18
Economic analysisThe economic analysis included a micro-costing study of ECP sessions based on time-driven activity-based costing (TDABC). In this method, resources are individually identified according to consumption, leading to a specific total cost per patient. Data collection for this individual assessment requires more effort, but provides accurate results that reflect the reality of the location in question.19
The TDABC has been presented in the literature as the most accurate method for assessing healthcare costs, supporting initiatives for the management of healthcare systems by value.20The fundamental principle of this method is to transform the cost drivers into time equations, which represent the time needed to carry out such an activity and, thus, measure the cost of services and/or products based on the actual consumption of resources. The TDABC method followed these steps: a) determine the details of the main activities that the patient undergoes; b) identify and calculate the costing of all resources consumed by the patient and the involved departments; c) develop time equations and allocate costs to activities, and; d) calculate the cost per patient.19
The capacity of the hemotherapy unit where the procedures took place was determined in hours and the unit's operating time with medical professionals was considered. To determine the professional capacity, the mean salary for each professional class in 2019 was considered. The 2019 hospital exam fees were considered, as was the 2016 purchase value of the ECP kit. The time required to conduct the sessions was determined by the observation of the procedure and interviewing the responsible physician and nurse. The data were compiled in an Excel® spreadsheet and the results are presented as absolute values.
Ethical considerationsAll patients indicated for the ECP therapy agreed to participate in this study and provide written informed consent. This project was approved by the research ethics committees of the Hospital Moinhos de Vento and the HCPA (number 63004716.6.1001.5330).
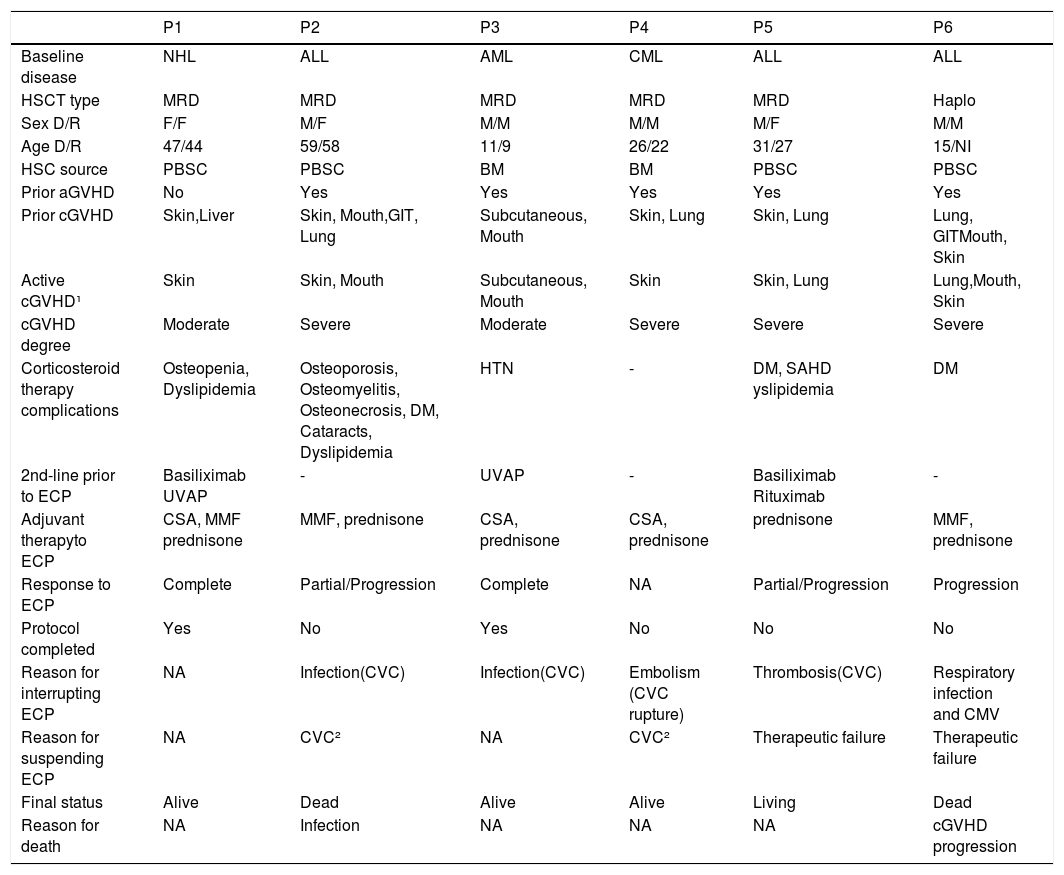
ResultsCase presentationSix patients who underwent ECP during the study period were included, four underwent HSCT at the HCPA and two underwent it at other centers. All of them were referred for ECP therapy to treat corticosteroid-dependent cutaneous cGVHD, of whom five already had complications due to corticosteroid therapy (Table 1). Prior to the ECP therapy, in addition to corticosteroids, these patients were using at least one immunosuppressant (cyclosporine, tacrolimus or mycophenolate mofetil). All of them had GVHD, which was severe in most cases, in at least two sites. (Table 1). Only one patient (P1) had a transient decrease in the hematocrit and hemoglobin related to the procedure, which required a transfusion of packed red blood cells. However, this did not impact the continuation of the ECP therapy. No patient experienced severe toxicity during treatment.
Clinical characteristics of the patients and ECP therapy.
NHL: Non-Hodgkin's lymphoma; ALL: acute lymphoid leukemia; AML: acute myeloid leukemia CML: chronic myeloid leukemia; HSCT: hematopoietic stem cell transplantation; MRD: matched related donor; Haplo: HLA-haploidentical donor; F: female; M: male; D/R: donor/recipient; HSC: hematopoietic stem cells; PBSC: peripheral blood stem cells; BM: bone marrow; NI: not informed; aGVHD: acute graft-versus-host disease; cGVHD: chronic graft-versus-host disease; GIT: gastrointestinal tract; ECP: extracorporeal photopheresis; DM: diabetes mellitus; HTN: systemic arterial hypertension; UVAP: psoralen with ultraviolet A phototherapy; NA: not applicable; CSA: cyclosporine; MMF: mycophenolate mofetil; CVC: central venous catheter; CMV: cytomegalovirus. ¹ The cGVHD was active at the time the ECP was indicated; ² CVC - related complications.
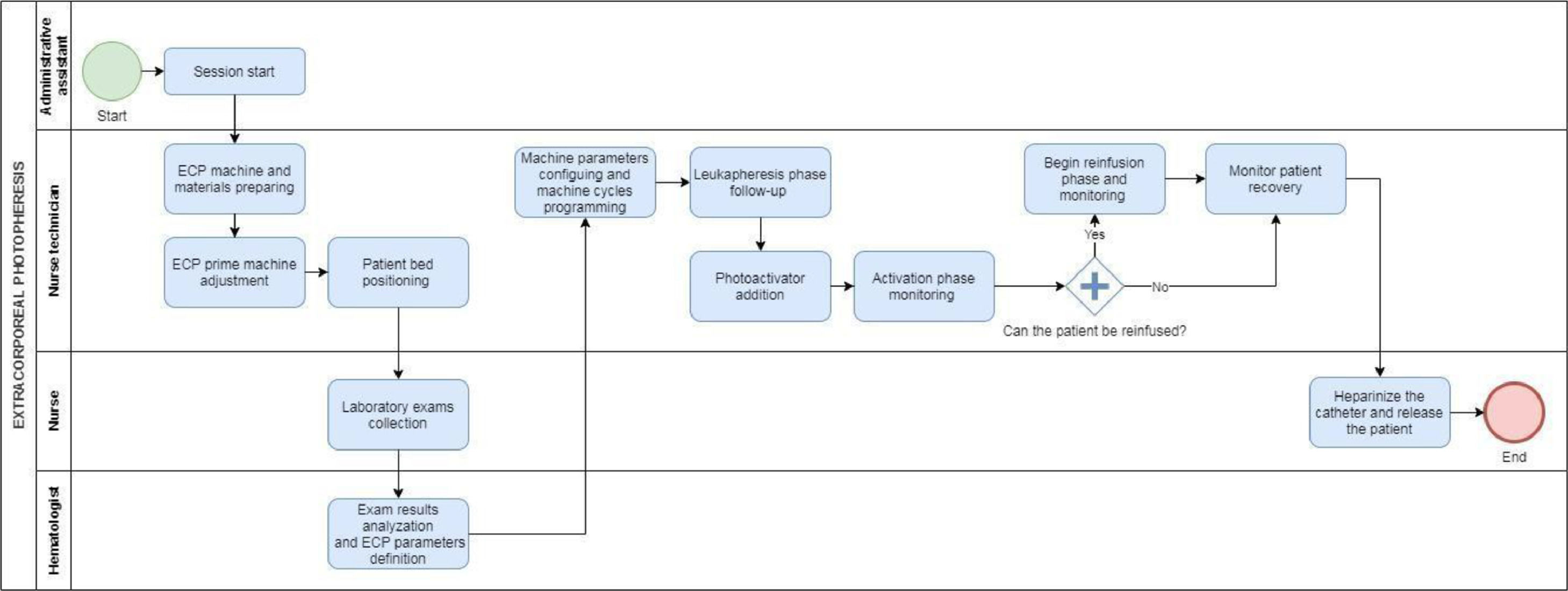
Through the observation of the steps of the procedure and interviews with the professionals involved in the process, the patient flow was designed, identifying the main procedures that patients underwent during each ECP session (Figure 1):
For each activity mapped, the resources involved were identified: professionals, unit structure, ECP kit, exams and supplies.
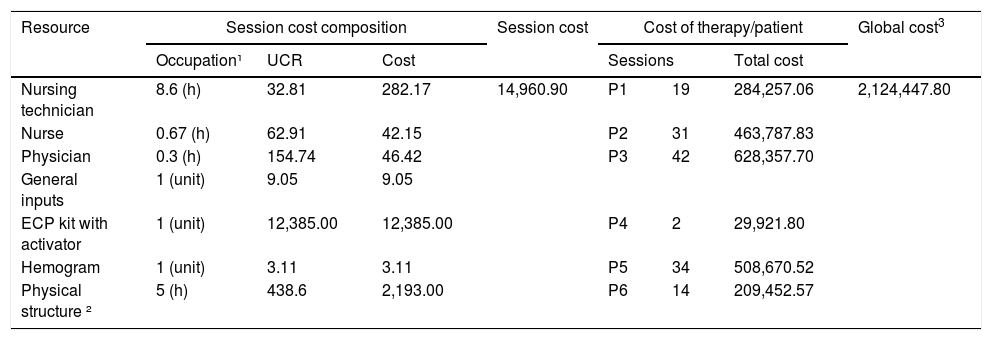
The ECP occurs in the hemotherapy unit and, according to the institution's estimates, its monthly cost is BRL 357,900.31. Based on this figure, the unit's monthly capacity was calculated by obtaining the cost per unit hour. The unit cost rate of BRL 438.60 was obtained by dividing the cost of the hemotherapy unit by the hourly capacity of the unit's physicians (816 hours/month) (Table 2).
The cost of ECP therapy.
Monetary values are presented in Brazilian reais (BRL). Legend: UCR: unit cost rate (BRL/h); Total cost: total therapy cost per patient. ¹Occupation refers to the use of the resource, either in hours (team/location) or in units (inputs); ²Physical structure refers to the Hemotherapy Unit; 3Global cost refers to the total ECP cost for all included patients.
The total session cost is obtained by multiplying the time of use by the hourly cost of each resource. For inputs, the consumption quantities were multiplied by the acquisition cost.
The cost per ECP session was BRL 14,960.90. This cost consists primarily of the ECP kit with an activator (82.78%), followed by the physical structure of the department (14.66%), human resources (2.48%) and exams/inputs (0.08%). The number of sessions per patient ranged from 2 to 42, and the cost of the complete treatment per patient ranged from approximately BRL 30,000 to BRL 500,000. The cost related to the staff training and the cost of acquiring Therakos™ UVAR® XTS™ were not considered.
DiscussionThis is the first micro-costing study to use the TDABC method to assess ECP costs. It was found that each session cost approximately BRL 15,000 and the response to treatment varied. Although it benefitted four patients, only two had a complete response and these patients had moderate global cGVHD without lung involvement.
To date, only one randomized clinical trial has assessed ECP in patients with GVHD refractory to/dependent on corticosteroids.13 In this trial, a ≥ 50% reduction in the corticosteroid dose and a ≥ 25% reduction in the total skin involvement were reported and this reduction was maintained even after the treatment interruption.13 Due to these benefits, this trial was interrupted in the 12th week and the ECP was also applied to patients in the control group, who had progressive improvement of skin/extracutaneous GVHD and reduced corticosteroid dose.14 These findings support the hypothesis that more prolonged ECP therapy may be more effective.
Regarding serious adverse events, Flowers et al. reported that there were serious complications in 28.6% of patients who underwent ECP, especially infection (18.4%), although there were no significant differences between the control and treatment groups. Other adverse events included anemia (24.5%), diarrhea (20.4%) and nausea (18.4%).13 There was only one case of a decrease in the hematocrit/hemoglobin concentration in our sample, which was transient and had no impact on the treatment. Three patients had CVC-related complications which culminated in the interruption/suspension of the ECP. Although the ECP can be performed through a peripheral access, the insertion of a CVC was necessary in all cases due to the profile of transplant patients who are submitted to chemotherapy/radiotherapy and multiple vascular punctures before the ECP. The described frequency of these complications is low, compared to those of long-term corticosteroid use.21
There have been no economic studies on the use of the ECP in Brazil. Only two cost-effectiveness studies with a similar clinical context have been published worldwide and they estimated the cost of the ECP sessions at € 1,125.50 and € 1,200.00.22,23 Despite the fact that there is less willingness to pay for the ECP in Brazil,24 it paradoxically has a higher cost based on current exchange rates.
From the introduction of the ECP therapy in 2016 until September 2020, only six patients underwent the process at our institution. This small sample is related to other options for second-line agents, the number of kits available and the previously mentioned high cost. Due to the limited number of kits, only the most difficult GVHD cases were referred for therapy, which may have impacted clinical response, as early initiation of treatment in refractory GVHD is related to better clinical response and favorable outcomes.25,26
The greatest impact on the cost of the ECP therapy is the activator kit itself, which represents 82.68% of the cost of each session. Other studies have also observed a high percentage of the cost of the ECP related to the activator kit. The total cost of the therapy varies according to the number of sessions necessary, which is related to the response. Since this response is variable, it is difficult to estimate the total cost of therapy per patient and plan institutional expenditures. However, these data should be analyzed with caution since other second-line therapies may have total costs similar to that of the ECP and greater associated toxicity.27,28,29 Considering this, it would be interesting to have a study comparing the cost of the ECP to that of new therapies strategies.
Understanding the total cost in the different stages of patient care in the HSCT, including post-transplant complications, such as the GVHD, contributes to actions in favor of improving care that can be initiated, considering the budget availability of the health system.19
Accurate calculation of costs is challenging and the lack of cost estimates in healthcare is a limiting factor in the development of economic evaluations in Brazil. Due to the use of different costing methodologies and cost systems, it is difficult to compare different health services.16 Thus, it is important to perform economic studies that contribute to the improvement of the health management system in Brazil, providing qualified information for decision-making. The limitations of the ECP in Brazil include the availability of sufficient structure and specialized human resources to perform the procedure and monitor the patient, in addition to the acquisition and maintenance cost of the ECP equipment. In addition, considering that the therapy cost is primarily due to the ECP kit with an activator, one possible strategy to reduce the cost of the procedure and make it more accessible would be to negotiate for a better price with suppliers and government representatives through larger scale purchases. European studies on the ECP cost present an activator kit cost approximately 3.5 times less than the acquisition cost in Brazil, considering the exchange rate on December 31, 2016 of 3.43 euros to one real.
Regarding the study limitations, the cost data collection from a single institution does not lend itself to extrapolation, as cost can vary between centers. However, as the main component of the ECP cost is the kit, it should not vary significantly between institutions. The limitations due to the small sample size are obvious, preventing a robust analysis of the treatment response. Nevertheless, the lack of standardization of a corticoid suspension protocol and the heterogeneity of the cases may have influenced the results. Despite these limitations, this study is essential, as it is the first to perform such an analysis in Brazil, discussing hypotheses that have so far been little explored. In addition, few centers in Brazil offer the ECP therapy and it is important to describe these cases. Thus, we believe that this study may assist in the resource management, as well as in the decision-making in the implementation of the ECP-based therapy for treating cGVHD at other HSCT centers.
ConclusionThe clinical response of cGVHD to ECP therapy was variable. The two patients with a complete response to the treatment had a moderate global GVHD score, without lung involvement.The cost analysis model presented here can be applied at other institutions that perform the ECP. The knowledge of accurate cost information on health treatments is essential to support cost control strategies in healthcare.