To analyze the effects of hospital cardiorespiratory physical therapy protocol on the functional capacity and quality of life of patients submitted to hematopoietic stem cell transplantation (HSCT).
MethodsFrom January to December 2019, bilateral dynamometry, Manovacuometry and Ventilometry, peak expiratory flow "Peak Flow", 6-min walk test (6MWT), SF-36 Quality of Life Questionnaire and Visual Analog Scale (VAS) were applied in patients who have undergone an allogeneic or autologous hematopoietic stem cell transplantation (HSCT), pre-conditioning (initial evaluation) and pre-discharge (final evaluation). The patients were submitted to an intervention protocol, consisting of aerobic training, muscle strengthening and respiratory muscle training, between the two assessments.
Results29 patients were enrolled in the study and 24 (83%) completed all procedure. Myeloablative and reduced intensity conditioning were performed in 89.6% and 10.4%, respectively; 17 (58%) patients have undergone an autologous HSCT; 10 (35%) identical related allogeneic HSCT, and 2 (7%) haploidentical allogeneic HSCT. The median number of interventions per patient was 3 (1–9). A decreasing in the right and left dynamometry (p ≤ 0.0001 and 0.002, respectively) and, also in the distance covered in the 6MWT (p = 0.004), was observed after HSCT. There was no significant difference in respiratory muscle strength, quality of life and fatigue sensation.
ConclusionCardiorespiratory rehabilitation can preserve functional capacity and quality of life.
Hematopoietic stem cell transplantation (HSCT) is widely used for the cure of a variety of hematological malignant and non-malignant diseases sustaining the quality of life and the functional capacity of the patients.1–3 Nevertheless, HSCT may also be associated with infection, respiratory, cardiovascular complications, poor nutritional performance, and loss of motor and respiratory muscle.4,5 Fatigue is related to the side effects of the conditioning regimen and the reduction of physical activity levels resulting from pancytopenia and their complications.6 During the conditioning period and until the engraftment, patients remain with restricted activities and in complete isolation.7 Physiotherapy provides measures and tools to evaluate the functional capacity and can improve the muscle strength and aerobic capacity.8,9 That support is fundamental for recovering functional deficits and earlier discharge.10 The aim of this study was to evaluate the effects of hospital cardiorespiratory physical therapy protocol in keeping the functional capacity and its impact in the quality of life of the HSCT patients.
MethodThis prospective and longitudinal study was developed at the HSCT Unit of the Clinics Hospital of University of Campinas, SP, Brazil, from January to December 2019. The project was approved by the ethics and research committee (CAAE no. 94927418.3.0000.5404).
Patients with hematological diseases and eligible for an autologous or allogeneic HSCT were invited to participate in the study. The patients elegible for an allogeneic HSCT were included if they had high or reduce dose allogeneic HSCT, with related HLA identical or haploidentical donors and graft source from bone marrow or peripheral blood. The conditioning regimens and GVHD prophylaxis were selected according to ongoing protocols at the University Hospital and patients were able to understand the proposed treatment and to sign the free informed consent.
The exclusion criteria were presence of neurological, cognitive and musculoskeletal disorders that might compromise the physical evaluation, hemodynamic instability, acute respiratory failure, sepsis and patients in contact isolation due to multidrug-resistant bacteria.
The presence of fever, gastro-intestinal tract abnormalities, dizziness or arterial pressure alteration was not exclusion criteria for the study. These conditions just delay the protocol application for a moment.
The functional evaluation, aerobic capacity and quality-of-life questionnaire were performed in two moments, pre-conditioning (initial evaluation), when patient was already hospitalized, and pre-discharge (final evaluation). The intervention proposed protocol was performed twice a week during hospitalization and included stretching, aerobic training, muscle strengthening of the upper limbs, and respiratory muscle. The following tools were applied to evaluate the functional and aerobic capacity: bilateral dynamometry (palmar grip strength),11 Manovacuometry (maximum inspiratory pressure and maximum expiratory pressure)12 and Ventilometry (measurements of tidal volume, minute volume and vital capacity)13 to evaluated the peak expiratory flow “Peak Flow”,14 and the 6-min walk test (6MWT).15 The questionnaire SF-3616 and the Visual Analog Scale (VAS)17 were applied to evaluate the quality-of-life.
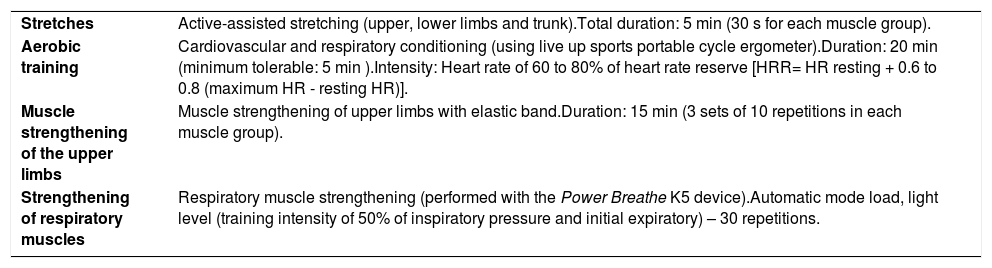
The intervention protocol is detailed in Table 1 and was initiated with global stretching followed by aerobic training in the portable ergometer cycle and muscle strengthening of the upper limbs with the use of an elastic band. The respiratory muscle training was also part of the protocol with the use of Power Breathe K5. The intervention exercises were performed according to Campanatti-Palhares Grando Protocol.18–20
Intervention protocol.
HRR: heart rate reserve.
All descriptive analysis and others were used in the IBM-SPSS (Statistical Package Social Sciences) version 24, considering p-value < 5%. The paired Student T-Test was applied, and when the assumptions were broken, it was used Independent T-Test.
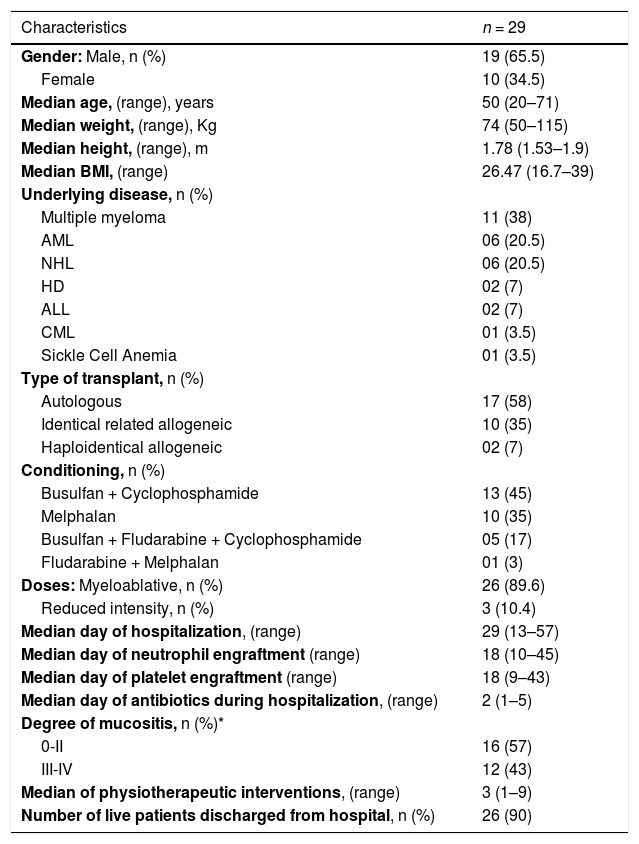
ResultsThe median age was 50 years (20–71) and, the majority of, patients were male (65.5%); 58% patients have undergone an autologous HSCT and 35% identical related allogeneic HSCT. 24 (83%) out of 29 patients completed the study, making possible to address the two evaluations had been designed. 5 out of 29 patients were not evaluated. 5 due to clinical complications (3 died). Table 2 depicts the patients characteristics.
Patients’ characteristics.
| Characteristics | n = 29 |
|---|---|
| Gender: Male, n (%) | 19 (65.5) |
| Female | 10 (34.5) |
| Median age, (range), years | 50 (20–71) |
| Median weight, (range), Kg | 74 (50–115) |
| Median height, (range), m | 1.78 (1.53–1.9) |
| Median BMI, (range) | 26.47 (16.7–39) |
| Underlying disease, n (%) | |
| Multiple myeloma | 11 (38) |
| AML | 06 (20.5) |
| NHL | 06 (20.5) |
| HD | 02 (7) |
| ALL | 02 (7) |
| CML | 01 (3.5) |
| Sickle Cell Anemia | 01 (3.5) |
| Type of transplant, n (%) | |
| Autologous | 17 (58) |
| Identical related allogeneic | 10 (35) |
| Haploidentical allogeneic | 02 (7) |
| Conditioning, n (%) | |
| Busulfan + Cyclophosphamide | 13 (45) |
| Melphalan | 10 (35) |
| Busulfan + Fludarabine + Cyclophosphamide | 05 (17) |
| Fludarabine + Melphalan | 01 (3) |
| Doses: Myeloablative, n (%) | 26 (89.6) |
| Reduced intensity, n (%) | 3 (10.4) |
| Median day of hospitalization, (range) | 29 (13–57) |
| Median day of neutrophil engraftment (range) | 18 (10–45) |
| Median day of platelet engraftment (range) | 18 (9–43) |
| Median day of antibiotics during hospitalization, (range) | 2 (1–5) |
| Degree of mucositis, n (%)* | |
| 0-II | 16 (57) |
| III-IV | 12 (43) |
| Median of physiotherapeutic interventions, (range) | 3 (1–9) |
| Number of live patients discharged from hospital, n (%) | 26 (90) |
BMI: body mass index; AML: acute myeloid leukemia; NHL: non-Hodgkin lymphoma; LH: Hodgkin's lymphoma; ALL: acute lymphoblastic leukemia; CML: chronic myeloid leukemia.
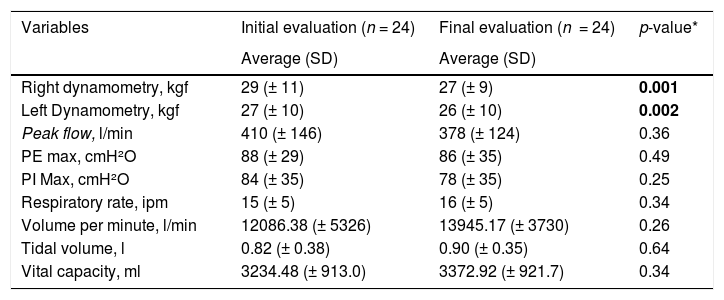
The median number of interventions per patient was 3 (1–9). All variables comparing the functional diagnosis before and after intervention were not statistically significant, but a significant decreasing in the right (29 versus 27, p ≤ 0.001) and left (27 versus 26, p ≤ 0.002) dynamometry were observed. Table 3 depicts the comparison of functional diagnosis before and after interventions.
Comparison between functional diagnosis before and after interventions.
| Variables | Initial evaluation (n = 24) | Final evaluation (n = 24) | p-value* |
|---|---|---|---|
| Average (SD) | Average (SD) | ||
| Right dynamometry, kgf | 29 (± 11) | 27 (± 9) | 0.001 |
| Left Dynamometry, kgf | 27 (± 10) | 26 (± 10) | 0.002 |
| Peak flow, l/min | 410 (± 146) | 378 (± 124) | 0.36 |
| PE max, cmH²O | 88 (± 29) | 86 (± 35) | 0.49 |
| PI Max, cmH²O | 84 (± 35) | 78 (± 35) | 0.25 |
| Respiratory rate, ipm | 15 (± 5) | 16 (± 5) | 0.34 |
| Volume per minute, l/min | 12086.38 (± 5326) | 13945.17 (± 3730) | 0.26 |
| Tidal volume, l | 0.82 (± 0.38) | 0.90 (± 0.35) | 0.64 |
| Vital capacity, ml | 3234.48 (± 913.0) | 3372.92 (± 921.7) | 0.34 |
Kgf: kilogram strength; l/min: liters per minute; PE max: maximum expiratory pressure; PI max: maximum inspiratory pressure; cmH²O: centimeter of water; ipm: inspirations per minute; l: liters; ml: milliliters.
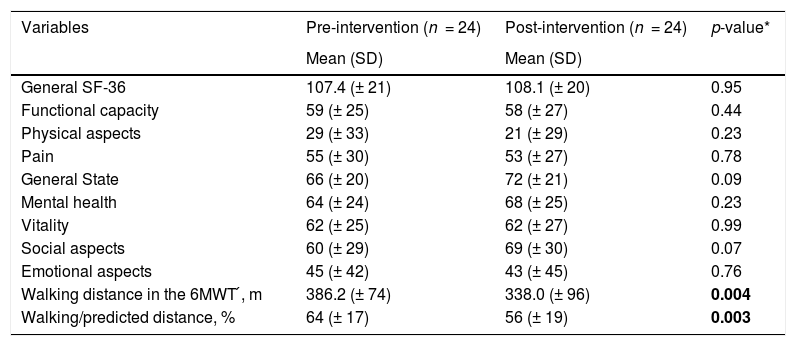
All variables comparing the domains of SF-36 and 6 MWT before and after intervention were also not statically significant, but the 6MWT (386,2 versus 338,0, p ≤ 0.004) and walking/predicted distance (64 versus 56, p ≤ 0.003) had significant decreases. Table 4 depicts the comparison of the SF-36 domains and the 6 MWT before and after intervention.
Comparison between the SF-36 domains and 6MWT before and after intervention.
| Variables | Pre-intervention (n = 24) | Post-intervention (n = 24) | p-value* |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| General SF-36 | 107.4 (± 21) | 108.1 (± 20) | 0.95 |
| Functional capacity | 59 (± 25) | 58 (± 27) | 0.44 |
| Physical aspects | 29 (± 33) | 21 (± 29) | 0.23 |
| Pain | 55 (± 30) | 53 (± 27) | 0.78 |
| General State | 66 (± 20) | 72 (± 21) | 0.09 |
| Mental health | 64 (± 24) | 68 (± 25) | 0.23 |
| Vitality | 62 (± 25) | 62 (± 27) | 0.99 |
| Social aspects | 60 (± 29) | 69 (± 30) | 0.07 |
| Emotional aspects | 45 (± 42) | 43 (± 45) | 0.76 |
| Walking distance in the 6MWT ́, m | 386.2 (± 74) | 338.0 (± 96) | 0.004 |
| Walking/predicted distance, % | 64 (± 17) | 56 (± 19) | 0.003 |
6MWT: six-minute walk test; m: meters; %: percentage.
Our study showed a reduction in bilateral palmar grip strength, post HSCT (p< 0.0001 for the right hand and p = 0.002 for the left hand), which may characterize the decrease in overall muscle strength, even with the application of the proposed protocol. The decrease in muscle strength, often related only to physical inactivity, can also be related to adverse symptoms related to conditioning and low adherence to exercises and the physical therapy intervention protocol applied was not able to improve the strength.7,21
Decreased tolerance to physical exercise after three weeks of HSCT, described by Morishita et al.,22 was also observed in our study that showed a decreasing in the walking distance evaluated by the 6MWT and walking/predicted distance, after HSCT. However, the perception of fatigue during exercise was similar before and after HSCT. These results might reflect the decrease in the cardiovascular and muscle conditioning, or low support, and the proposed protocol was not able to improve them.23 Possibly, if the number of sessions were higher, increasing the intensity of the exercises, the results might be more evident. Fatigue and loss of strength in performing daily life activities are symptoms of functional deficit and those results can be related to the prognosis after HSCT.24
The non-significant differences in the values of the pre- and post-HSCT evaluation may suggest that the physical therapy protocol can prevent the worsening of the functional capacity loss after HSCT because, there are data showing a significant reduction in the functional capacity after HSCT.4,25,26,27
The patients in whom our proposed protocol was applied, no significant difference in the SF-36 domains before and after intervention was observed showing a preservation of quality-of-life. A tendency in a higher mean value of the domain “General State of Health” [pre 66 (SD± 20) and post 72 (SD± 21), p = 0.09)] was observed. This domain reflects the patients’ perception of their health, which is his/her perspective of improvement.28
In addition, the exercises performed were not related to any adverse clinical effects. The presence of pancytopenia should not be a factor of contraindication to physical exercise. This activity can be performed safely, respecting the intensity tolerated by the patient, as stated by Morishita et al.29
A potential risk associated with the intervention was a concern. To prevent those risks patients during the exercises were monitored in order to keep the heart rate in 60 to 80% of resting heart rate, peripheral oxygen saturation greater than or equal to 90%, and systolic blood pressure between 90 to 140 mm of mercury and diastolic blood pressure between 60 and 90 mm of mercury.18–20 The median number of interventions per patient was 3 (1–9) reflecting the clinical condition complexity associated by the presence of fever, gastrointestinal discomfort, pain, blood pressure changes, platelet number less than 10,000/mm³ and hemoglobin below 8 g/blood deciliter which hampered a higher number of interventions sessions.
One limitation of our study is the heterogenicities related to underlying diseases, as well the type of HSCT. Nevertheless, 90% of the evaluated patients received a myeloablative conditioning making it a homogeneous population regarding outcome risks. Moreover, the sample size was not applied for obtaining adequate statistical power. Due that, our sample was not probabilistic and the enrolled eligible patients were consecutives and based on the availability for HSCT during the study period avoiding bias inclusion.
ConclusionCardiorespiratory rehabilitation can preserve functional capacity and quality-of life. We have not reported significant functional loss when was compared between the initial and final evaluation.
We conclude that is possible to perform safely hospital cardiorespiratory therapy protocol during the HSCT, even in pancytopenia conditions.