Regarding the close association between neonatal hyperbilirubinemia and occurrence of pathological jaundice as a cause of neurotoxicity and kernicterus, the present study aimed to evaluate the use of intravenous immunoglobulin (IVIG) in neonates with hyperbilirubinemia.
MethodsA retrospective case-control study of blood group O mothers and their ABO and Rh newborns was conducted. Medical records that included total serum bilirubin levels of 79 patients with hemolytic disease of the newborn (HDN) from between 2017 and 2020 were reviewed. Neonates who were eligible to receive immunoglobulin based on the American Academy of Pediatrics (AAP) guidelines were classified as cases and the rest were included as the Control Group.
ResultsThe mean total bilirubin in relation to hemoglobin levels in IVIG-treated neonates was significantly lower than in non-IVIG-treated neonates (13.98 ± 4.23 mg/dL versus 16.61 ± 2.68 mg/dL; p-value = 0.002). Although females had longer hospitalizations in both IVIG-treated (3.81 ± 1.28 versus 3.54 ± 1.30 days; p-value = 0.509) and non-IVIG-treated (3.43 ± 0.811 versus 3.19 ± 0.75 days; p-value = 0.361) groups compared to males, this difference was not significant between the groups. Although four neonates with ABO incompatibility required packed red blood cells, all infants were managed medically and no deaths occurred during the course of treatment. Moreover, no exchange transfusion or adverse effects of IVIG were observed.
ConclusionThe results from the present study revealed that IVIG administration is a useful procedure for the management of bilirubin encephalopathy with greater opportunity to reduce exchange transfusion requirements for neonatal hyperbilirubinemia.
Hyperbilirubinemia is one of the most common clinical problems of newborns especially in the first week of life; it is observed in approximately 60 % and 80 % of full-term and preterm infants, respectively.1-3 The yellow color of the skin and mucous membranes, called jaundice, is due to the accumulation of unconjugated bilirubin (UCB) pigment.4,5 The risk factors of hyperbilirubinemia are numerous and include cephalohematoma, prematurity, unsuccessful breastfeeding and immunologic or non-immunologic anemia6.
Generally, jaundice is of physiological, pathological, hemolytic and breast milk etiology. Physiological jaundice occurs as a limited ability to eliminate bilirubin by the liver in the first few days of life and is not related to red blood cell destruction. Due to the antioxidant properties of bilirubin, this increase at the beginning of life can protect the baby from the high concentrations of oxygen in the air.7,8 Diagnosis of physiological jaundice in preterm and term infants is based on the exclusion of other causes of jaundice based on history, tests and examinations.9,10 Jaundice is considered pathological if the time, duration, or pattern of onset is significantly different from physiological jaundice. The most important pathological causes of hyperbilirubinemia are immune and non-immune hemolytic anemia, sepsis and the presence of bruising or other extravasation of blood in which the infant may be at risk for bilirubin-induced brain injury.3,11 Although several factors such as central nervous system maturation at the time of exposure, hemolysis, inflammation, acidosis and some genetic factors are involved in the pathology of bilirubin-induced brain injury, the exposure of astrocytes and neurons to free bilirubin has a pivotal role through impairment of energy metabolism, decreasing oxygen consumption of brain cells and death.12,13
The goal of jaundice treatment is to prevent kernicterus spectrum disorder due to hyperbilirubinemia. Indeed, UCB penetrating the central nervous system affects many neuronal and non-neuronal cells. One class of these cells is microglia which secrete many pro-inflammatory cytokines and matrix metalloproteinases (MMPs).12,14 MMPs are calcium-dependent zinc-containing endopeptidases that digest various extracellular matrix (ECM) macromolecules which have a role in modulating bilirubin-induced neurotoxicity.13 Neonatal jaundice treatment includes phototherapy, exchange transfusion (ET) and, alternatively, intravenous immunoglobulin (IVIG) therapy.15 Phototherapy is at the forefront of therapeutic interventions and is the most widely used method of treatment. In the event of intensive phototherapy failure, ET is the second most widely used treatment method.16 Some documented researches have shown that the treatment of infants with IVIG, especially those with a blood type incompatible with their mother's, significantly reduces the need for ET.17,18 Contrariwise, some studies questioned the effect of IVIG on improving the condition of infants with jaundice.19,20
The current study was conducted to investigate the effect of IVIG on hyperbilirubinemia in neonates with neonatal jaundice and the need for immediate treatment under certain conditions with the introduction of IVIG as a new treatment in these infants,
Material and methodsThis retrospective cross-sectional study was performed on neonates with jaundice who were admitted to the 17-Shahrivar Hospital in Rasht, Iran during the period of 2017–2020. According to the 2004 American Academy of Pediatrics (AAP) guidelines, IVIG infusion can be used in neonates with hyperbilirubinemia due to hemolytic disease of the newborn (HDN) particularly if the total serum bilirubin is rising despite intensive therapy or if the bilirubin level is within 2–3 mg/dL (34–51 mmol/L) of the threshold level for ET.21 Those neonates who were eligible to receive IVIG based on the AAP guidelines were classified as cases and the rest were included in the Control Group.
The case group included healthy term and near-term neonates (gestational age ≥35 weeks) who were diagnosed with jaundice based on clinical and laboratory evidence including total serum bilirubin, direct antiglobulin test (DAT), direct serum bilirubin, hematocrit, complete blood count, glucose-6-phosphate dehydrogenase screening, blood culture and liver function tests. Although, the parameter used was total bilirubin as long as direct bilirubin was normal, in situations where the direct bilirubin level was more than 2 mg/dL or 20 % of the total serum bilirubin (conjugated hyperbilirubinemia), the serum level of indirect bilirubin was calculated by the difference between total and direct bilirubin.22
The demographic characteristics, including sex, age, gestational age, IVIG therapy, length of phototherapy, blood transfusions and medical complications were collected through a review of the medical records.
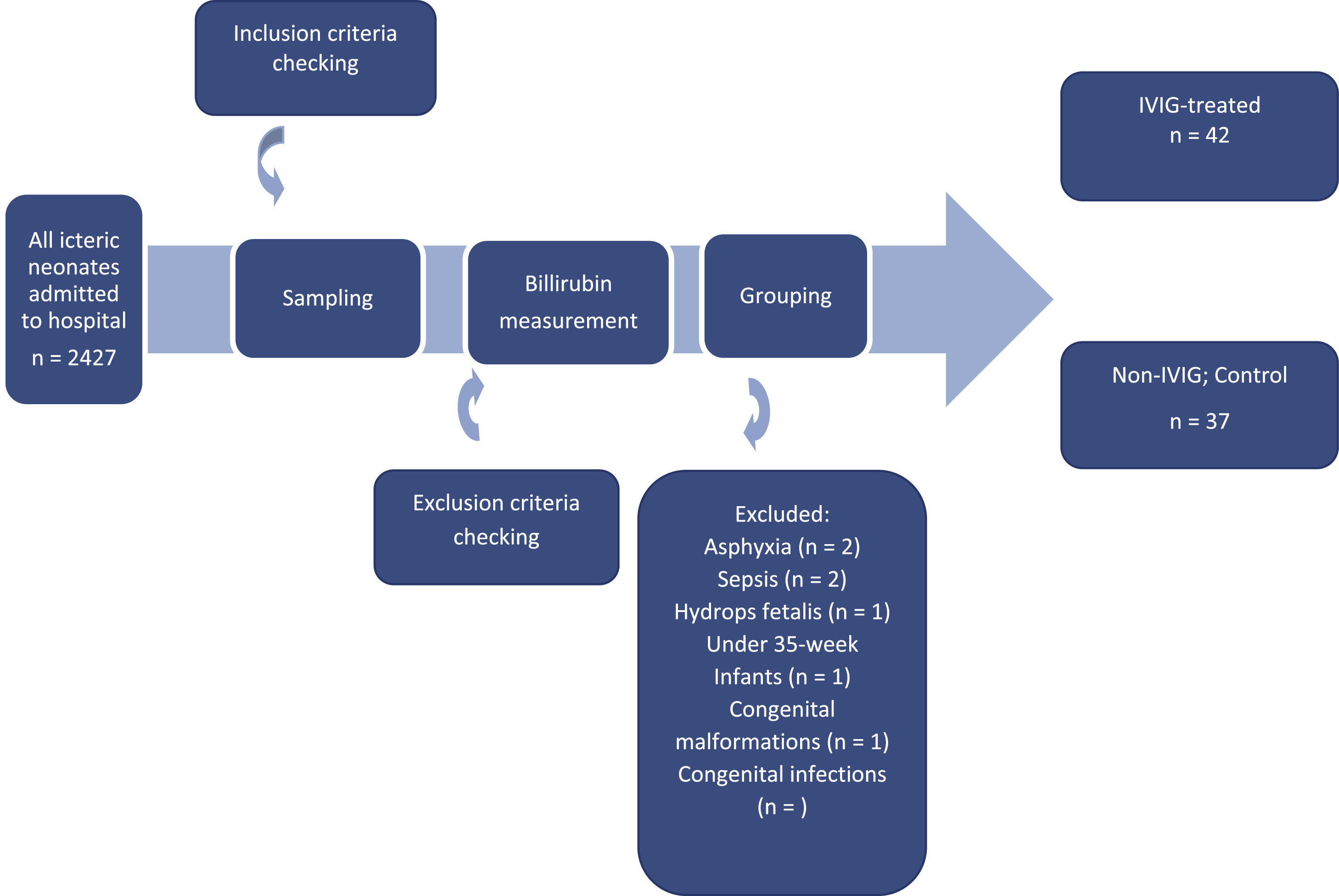
Neonates with a history of asphyxia, sepsis, hydrops fetalis, thrombocytopenia, congenital infections, congenital malformations, infants with <35 weeks gestational age, birth weight <2500 g, the presence of hematomas or diffuse cutaneous bruising and from mothers with other ABO blood groups except type O were excluded.
Phototherapy was initiated when the newborn's serum bilirubin level reached the phototherapy threshold and stopped when the bilirubin levels dropped to safe limits according to the AAP criteria.21 Phototherapy was provided using a LED phototherapy system (Tosan Medical Inc., Tehran, Iran) providing 32 microW/cm2.nm intensity. In the IVIG group, immunoglobulin treatment with phototherapy was started in neonates with HDN based on the aforementioned guidelines.
Hemolysis was diagnosed by anemia (hemoglobin level below 14 g/dL), reticulocytosis (reticulocyte count above 7% on the first day, above 3% between second and fourth days and above 1% by 7 days of life) and abnormal peripheral blood smear with spherocytes, polychromasia and increased number of nucleated red blood cells.6 IVIG would be administered when the neonate's bilirubin value was only 2–3 mg/dL below the threshold for ET. Possible side effects of IVIG such as fever, anaphylaxis, bronchospasm, hemolysis, necrotizing enterocolitis, tachycardia were recorded and the bilirubin was measured in all newborns before discharge.
This study was carried out in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of Guilan University of Medical Sciences and informed consent waiver was obtained as it is a retrospective analysis. (Ethical Code: IR.GUMS.REC.1398.445).
Statistical analysisFor statistical analysis, the results are presented as means ± standard deviation (SD) for quantitative variables and are summarized by absolute frequencies and percentages for categorical variables. Categorical variables were compared using the chi-square test or Fisher's exact test. Between groups, quantitative variables were also compared with the t-test or Mann-Whitney U test. Correlations between continuous data used Spearman's correlation coefficient. For the statistical analysis, the statistical software Statistical Package for Social Sciences (SPSS) version 16.0 for windows (SPSS Inc., Chicago, IL) was used. P-values of 0.05 or less were considered statistically significant.
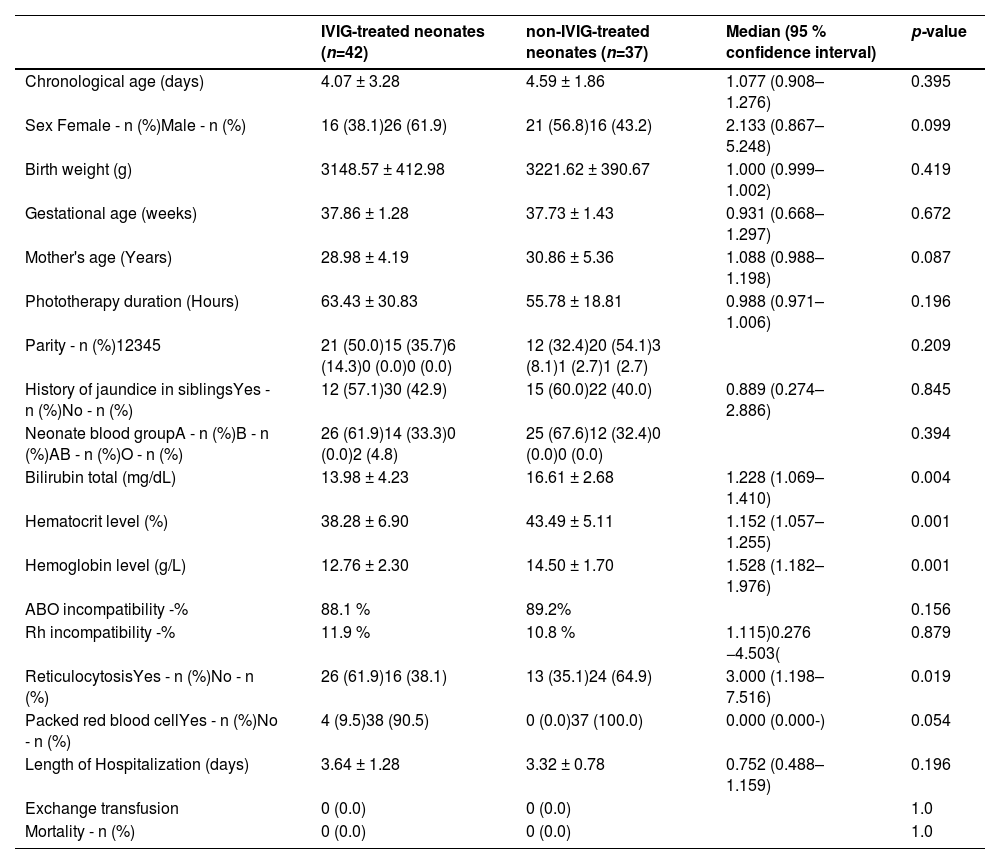
ResultsA total of 79 neonates were included in the study on the basis of the inclusion criteria they received phototherapy immediately after admission to the neonatal ward (Figure 1). The reason for the exclusion of neonates included other causes of neonatal jaundice such as breast milk jaundice, prolonged jaundice, excessive physiological jaundice, etc. Furthermore, a number of infants required ETs from the time they were admitted to the hospital or were not candidates for IVIG according to the AAP guidelines. Finally, infants with mothers with other ABO blood groups except type O were excluded. As summarized in Table 1, there was no significant difference in the demographic characteristics, including chronological age, sex and birth weight, between the two groups with or without IVIG therapy. The mean of total bilirubin in IVIG-treated neonates was significantly lower than in non-IVIG-treated neonates (13.98 ± 4.23 mg/dL versus 16.61 ± 2.68 mg/dL: p-value = 0.004).
Demographic, clinical and outcome characteristics in IVIG-treated and non-IVIG-treated neonates.
All mothers of both IVIG and non-IVIG-treated infants had the O blood group type. Between the two groups, 51 neonates had blood group A, 26 had blood group B and two had blood group O. There were no statistically significant differences between the two groups with respect to their blood groups (Table 1). Here, 95.2 % in the IVIG-treated group and 100.0 % in the non-IVIG-treated group had no compatibility of ABO blood group system between mother and child (median: 0.952; 95 % confidence interval: 0.890–1.019; p-value = 0.179). Among them, 88.1% of IVIG-treated and 89.2% of non-IVIG-treated neonates had just ABO incompatibility and there was no statistically significant difference between the groups (p-value = 0.156).
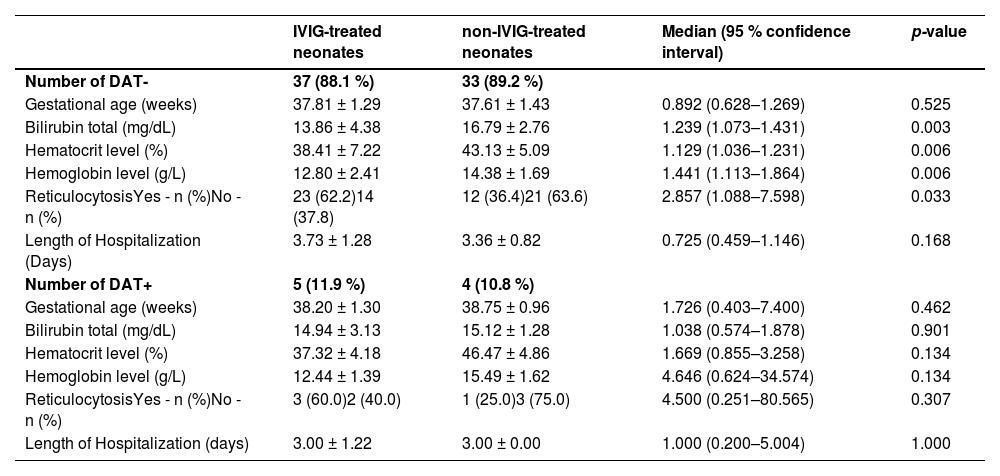
According to the findings of this study, 22 (59.5%), 13 (35.1%) and 2 (5.4 %) neonates in the IVIG-treated group, as well as 23 (65.7%), 12 (34.3%), and 0 (0 %) neonates in the non-IVIG-treated group were Rh+ with blood group A, B and O, respectively. Rh incompatibility was present in 11.9% of the IVIG-treated and 10.8% of non-IVIG-treated neonates; in respect to this there was no statistically significant difference between the groups (median: 1.115; 95 % confidence interval: 0.276–4.503; p-value = 0.879). Mean serum bilirubin levels were significantly higher in non-IVIG-treated neonates compared to those treated with IVIG in both DAT negative (16.79 ± 2.76 mg/dL versus 13.86 ± 4.38 mg/dL; p-value = 0.003) and positive groups (15.12 ± 1.28 mg/dL versus 14.94 ± 3.13 mg/dL; p-value = 0.901 – Table 2).
Baseline characteristics and outcome in IVIG-treated and non-IVIG-treated neonates with Rh incompatibility.
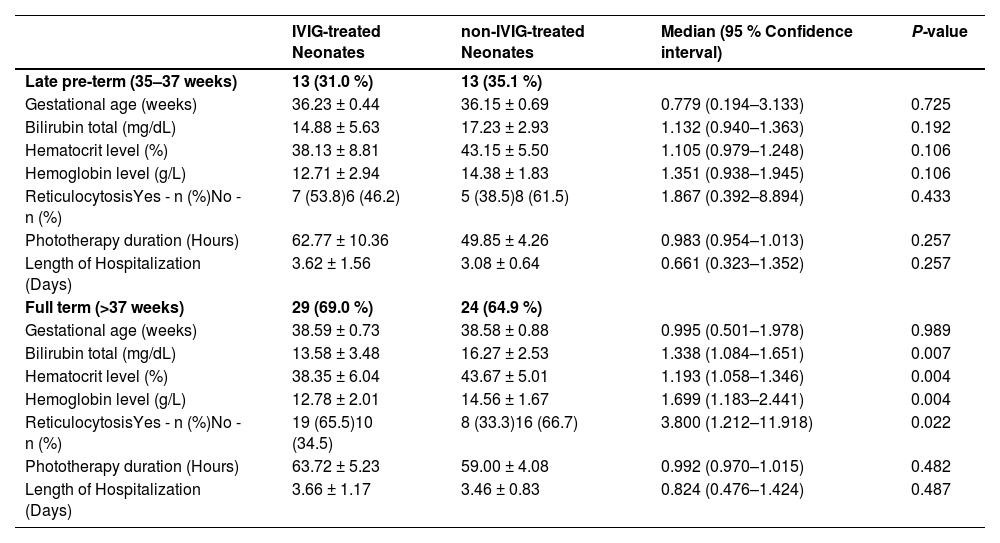
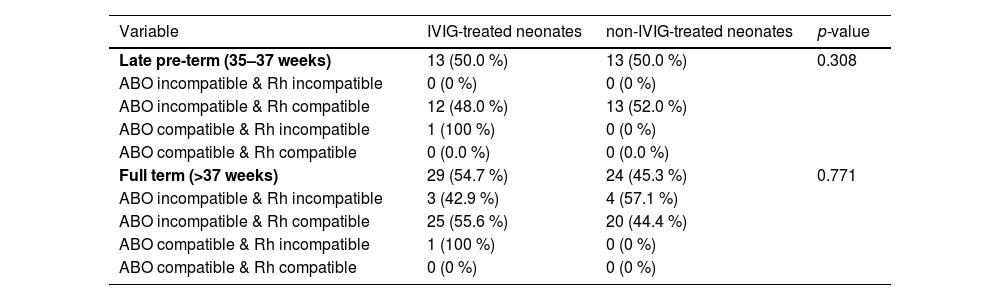
Considering the gestational age of neonates, 79 infants were classified as late pre-term (n = 26; 32.9 %) and full-term neonates (n = 53; 67.1 %; p-value = 0.693). Based on Table 3, there was no statistically difference between the two groups with or without IVIG therapy in respect to total bilirubin, hematocrit or hemoglobin levels in the late pre-term groups. However, the mean total bilirubin in IVIG-treated neonates was significantly lower than that in non-IVIG-treated neonates in full-term neonates (13.58 ± 3.48 mg/dL versus 16.27 ± 2.53 mg/dL; p-value = 0.007).
Baseline characteristics and outcomes in IVIG-treated and non-IVIG-treated neonates based on gestational age.
Females had longer length of hospitalization (LOH) in both IVIG-treated (3.81 ± 1.28 days versus 3.54 ± 1.30 days; p-value = 0.509) and non-IVIG-treated (3.43 ± 0.811 days versus 3.19 ± 0.75 days; p-value = 0.361) groups compared to males, however, there were no significant differences in the LOH between groups. Moreover, IVIG-treated neonates had longer LOH in both late pre-term (3.62 ± 1.56 days versus 3.08 ± 0.64 days; p-value = 0.257) and full-term (3.66 ± 1.17 days versus 3.46 ± 0.83 days; p-value = 0.487) groups compared to non-IVIG-treated neonates, nevertheless there were no significant differences in the LOH between groups. Such a trend was also been shown by phototherapy duration and ABO and Rh compatibility in both Late pre-term and full term groups (Tables 3 and 4).
ABO and Rh compatibility in IVIG-treated and non-IVIG-treated neonates based on gestational age.
No statistically differences between Late pre-term (35–37 weeks) and Full term (>37 weeks) p-value = 0.879.
Here, 64.9 % of neonates with just ABO incompatibility received one dose and 35.1 % two doses of IVIG compared to ABO plus Rh incompatible neonates, 66.7 % of whom received one dose and 33.3 % received two doses of IVIG; there was no statistically difference between these groups. Additionally, no adverse effects of IVIG, including type I hypersensitivity (anaphylaxis), thromboembolism, renal insufficiency or atypical meningitis except tachycardia (7.1 %), was observed.
DiscussionThe most prominent organ involved in neonatal hyperbilirubinemia is the brain. To reduce morbidity and mortality, the serum bilirubin level is usually used as an indicator in diagnosis; when increased it leads to jaundice. The mainstay of jaundice treatment is intensive phototherapy followed by ET, but recent advances in pharmaceutical developments have produced medications including IVIG that modulate bilirubin encephalopathy in response to severe hyperbilirubinemia.23,24
Here, the effect of IVIG was examined in 79 neonates with hyperbilirubinemia divided in two groups to find a better approach to modulate this problem. It is reported that about 30% of blood group A or B neonates born to a blood group O mother will have a positive DAT, but our data showed nine neonates (11.39 %) with Rh incompatibility in three years. Currently, chances of primary sensitization during the first pregnancy is about 1 % because of anti-Rh gamma globulin administration to Rh– pregnant women based on their indirect antiglobulin test at around week 28 of pregnancy and again within 72 h of birth.25
Carboxyhemoglobin studies revealed that IVIG infusion in DAT-positive neonates reduces hemolysis which is in agreement with the findings of this study that there is no statistically difference in reticulocytosis in neonates with anti-D alloimmunization.26 Based on the findings of the present study there is no significant difference in the LOH in neonates with anti-D alloimmunization (Table 2). Although some studies reported that IVIG reduced the duration of phototherapy and hospitalization, others reported no benefit in outcomes.20,27
Besides, the LOH in neonates with negative DAT in the phototherapy and phototherapy plus IVIG groups was higher compared to positive DAT neonates. Agrawal et al. reported the role of the UGT gene polymorphism in the severity of neonatal hyperbilirubinemia in DAT-negative infants which might partly explain this disparity between infants with positive and negative DAT.28
According to the findings of this study, the mean total bilirubin in IVIG-treated neonates was significantly lower than in non-IVIG-treated neonates. Considering the significant drop in hemoglobin and hematocrit, and high reticulocytosis due to severe hemolysis in neonates in the IVIG group compared to the Control Group, according to the APP protocol, treatment at low levels of bilirubin in the first days of life will be required. This describes the lower levels of bilirubin in the IVIG treatment group compared to the Control Group.21
Type O blood group mothers whose serum contains predominantly IgG rather than IgM antibodies against A and B antigens were included in this study to define the impact of the mothers immunoglobulin isotype which can cross the placenta. Indeed, about 15–20 % of pregnancies involve an O type mother and a non-O neonate; unlike Rh incompatibility, this can occur in the first pregnancy. Only 1 % of ABO incompatibility cases result in hemolytic disease.27 In this study, there was no statistically significant difference between the groups in this regard. In parallel with previous research, administering IVIG when the neonate's bilirubin level is 2–3 mg/dL below the threshold has avoided ET in neonates with ABO isoimmune hemolytic disease.26 However, Beken et al. reported that IVIG therapy did not decrease neither phototherapy nor hospitalization duration in infants with ABO hemolytic disease.18
IVIG is an immunomodulating agent with multiple activities, including modulation of complement activation and suppression of various inflammatory mediators. Moreover, the saturation of immunoglobulin Fc receptors on macrophages prevents the destruction of neonatal red blood cells by transplacental transfer of maternal antibodies including anti-A, anti-B and anti-A,B as an antibody with significant affinity for both group A and group B antigens, which reacts with A and B cells. The AAP recommended IVIG therapy for hyperbilirubinemia of neonates21,25 nevertheless, judgment of its effectiveness in these neonates should take into account the criteria for accepted goals from duration of hospitalization to exchange requirement.
In various published resources, there is an uncertainty about the amount and frequency of IVIG administration; low-dose IVIG (0.5 g/kg) reduces the duration of hospitalization without causing complications, but it is not as effective as high doses (1 g/kg) in reducing the amount of blood exchange.29,30 Moreover, in some articles, multiple doses have shown greater advantages than single administration in regard to ET in ABO and Rh HDN31,32 whereas, other authors reported no obvious benefit of IVIG therapy.33 No statistically significant difference was observed between the two groups in this study.
The major challenge in the current study is the relatively small number of patients despite of three years investigation. Further studies about the administration of IVIG are required to generalize the findings to broader populations. IVIG therapy in infancy has several complications, such as hemolysis and necrotizing enterocolitis.27 Based on the data of this study, there were no significant side effects in neonates except tachycardia. Although, four neonates with ABO incompatibility, equally of both sexes, required packed red blood cells, all infants were managed medically and no mortality occurred during the course of treatment.
ConclusionsCollectively, the results of the present study revealed a reasonable decrease of the necessity of ET in HDN. These patients are at high risk for acute bilirubin encephalopathy and kernicterus spectrum disorder. Administration of IVIG is a useful procedure in the management of jaundice with greater opportunity to reduce ET for neonatal hyperbilirubinemia.
Funding statementThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributionsSZJ: Conceptualization, Supervision, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. SM: Methodology, Writing – review & editing. RA: Data curation, Investigation, Methodology, Writing – review & editing. FS: Formal analysis, Visualization, Writing – original draft, Writing – review & editing.
Ethical approval statementThe study was approved by the Ethics Committee of Guilan University of Medical Sciences and informed consent waiver was obtained as it is a retrospective analysis. (Ethical Code: IR.GUMS.REC.1398.445).
Data availabilityThe data pertaining to the study is available with the corresponding author.
The authors acknowledge the contribution of all our participants for their generous assistance during the project study.