The demand for apheresis platelets has increased in the recent past and the shrinking donor pool has shifted the trend to collection of double-dose or higher yield of platelets.
ObjectiveThe present study aimed to determine the effect of double-dose plateletpheresis on the target yield and donor platelet recovery.
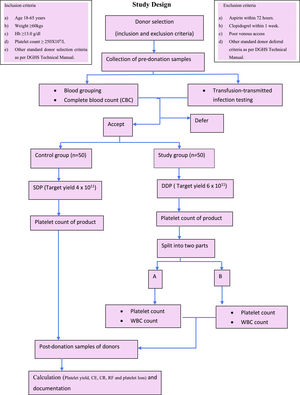
MethodsThe study was conducted on 100 healthy plateletpheresis donors, 50 of whom were in the study group, which underwent double-dose plateletpheresis (DDP), and 50 of whom were in the control group for single-donor plateletpheresis. Pre- and post-procedure samples of donors were subjected to a complete blood count. The DDP product was sampled for platelet yield and then split into two parts. Platelet yield, collection efficiency, collection rate, recruitment factor and donor platelet loss were calculated.
ResultsThe mean platelet yield in the SDP was 4.09 ± 1.15 × 1011 and in the DDP, 5.93 ± 1.04 × 1011. There was a significant correlation between the pre-donation platelet count and platelet yield. The total of platelets processed for the SDP were 5.42 ± 1.08 × 1011 and for the DDP, 7.94 ± 0.77 × 1011. The collection efficiency was 71.93 ± 25.14% in the SDP and 72.94 ± 16.28% in the DDP, while the collection rates were 0.78 × 1011 and 0.94 × 1011 per minute, respectively. The average recruitment factor observed was 0.98 in the SDP, while it was 0.99 in the DDP. The mean platelet loss observed in the SDP was 35.55 ± 8.53% and in the DDP, 37.76 ± 8.65%.
ConclusionThe double-dose plateletpheresis supplements the platelet inventory in developing countries where the apheresis donor pool is limited. It is prudent to ensure stringent donor selection criteria for donors donating high-yield platelet products, thus enhancing donor safety and retention.
Plateletpheresis is the procedure of collecting platelets from the donors using automated apheresis machines known as single donor platelets (SDPs). With the advent of newer techniques of component extraction and newer generation apheresis platforms with better efficiency for platelet collection, it is now considered as an integral part of modern transfusion practice.1 The increasing trend in platelet demand due to the rising number of patients with various bleeding manifestations and also developments in medical sciences, including transplantation programs, has significantly escalated the demand for platelets (PLTs).2,3 Due to availability of SDPs, the treatment of patients with oncological and hematological disease and bone marrow transplantation has improved, as it provides an efficient means of platelet support from a single donor, minimizing platelet transfusion refractoriness.4 Apart from the greater dose, SDPs are leukodepleted, which further helps in the prevention of transfusion reactions, such as the febrile nonhemolytic transfusion reaction (FNHTR), human leukocyte antigen (HLA) alloimmunization and transmission of infectious agents, which are more associated with random donor platelets (RDP).5
Storage of platelets at room temperature poses a risk for bacterial contamination. The shelf life of platelets is limited to five days and the selection of plateletpheresis donors is based on stringent eligibility criteria, which is crucial in maintaining an adequate platelet inventory. Due to the shrinking donor pool and increasing demand for single donor platelets, the practice of collecting a double dose of platelets or even higher yield is on the rise. This is possible due to the newer generation apheresis platforms with better efficiency for platelet collection. This further helps in reducing the cost, risk of multiple donor exposure and adverse transfusion reactions associated with allogeneic transfusion.6
The standards prescribed by the American Association of Blood Banks (AABB) mandates that SDPs should have at least 3.0 × 1011 platelets in each unit. With modern mechanical blood cell separators, the collection of the double dose and triple dose of platelets from a single donor is feasible, but each unit collected from these must meet the quality control criteria, as per prescribed standards.7 Donor safety is of prime importance in plateletpheresis donors to avoid high platelet loss and prevent adverse donor reactions, but there is a paucity of data for the same in the acquisition of higher yield products. The present study aimed to analyze the effect of double-dose platelet (DDP) extraction on platelet product quality and donor platelet recovery.
Materials and methodsThe current study was a prospective observational study conducted in the Department of Transfusion Medicine of a tertiary care center. The study was undertaken after receiving the approval of the Institutional Research and Ethics Committee. Informed written consent was obtained from all the donors.
A total of 100 healthy plateletpheresis donors (both voluntary and replacement) eligible for donation and willing to participate in the study were included. The subjects were divided into two groups:
Study group: Donors fulfilling criteria for DDP collection (n = 50).
Control group: Donors eligible for SDP collection (n = 50).
All donors (first time or repeat) meeting the general eligibility criteria for plateletpheresis established by the Drug Controller General of India and the Director of General Health Services (DGHS) guidelines were considered for enrolment in the study.8,9 In addition, donor selection for DDP extraction was based on additional criteria, such as weight ≥ 60 kg, Hemoglobin (Hb) ≥ 13.0 g/dL, platelet count ≥ 250 × 109/L, previous plateletpheresis more than one month beforehand or whole blood donation more than 3 months previously. The platelet count cutoff for the control group (SDP) was ≥ 150 × 109/L. A pre-donation sample from each donor was subjected to a complete blood count (CBC) using automated cell counter (Cell Tech Automated Cell Counter, Rome, Italy), blood grouping by tube method and screening for transfusion transmitted infection (TTI) markers anti-HIV 1 and 2, anti-HCV, HBsAg, syphilis and malaria, as per departmental standard operating procedure.
The plateletpheresis procedure was performed on the Amicus cell separator (version 2.5) using single-needle closed-system apheresis kits for both SDP and DDP collection. The anticoagulant acid citrate dextrose (ACD) infusion rate was 1.25 mg/kg/min, the maximum inlet rate varied from 45 ml/min to 150 ml/min. The endpoint was the target yield set. The target yield for each procedure in the study group was ≥ 6.0 × 1011 and in the control group, between 3 × 1011 and 4 × 1011. The collection time, processed blood volume, ACD used, product volume, post platelet count, post hematocrit (Hct) and total saline used was recorded at the end of each procedure.
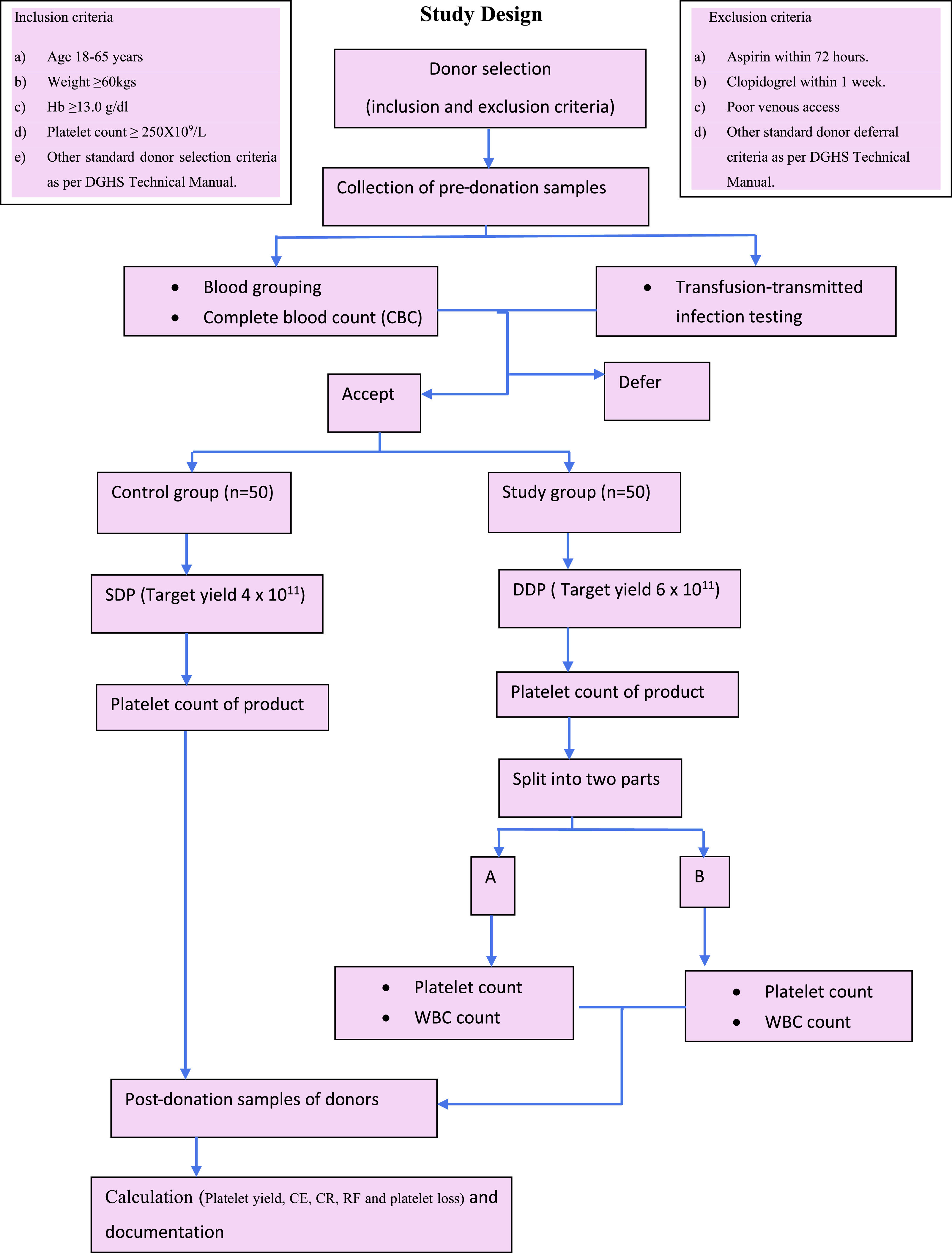
After the completion of the procedure, the donor rested for 15–20 min and a 2 ml post-donation sample of the donor was collected in an EDTA vial and subjected to a complete hemogram. The platelet product was rested for one hour for proper disaggregation before sampling. The sample was taken from the primary bag and tested for platelet content. The final product of the study group was then split into two equal parts using a weighing scale and labelled as A and B and, as described above, representative samples were taken from each bag and subjected to platelet count and leucocyte count on an automated hematology analyzer. The sample was collected after ensuring thorough stripping of the attached segment. The final volume of each bag, both A and B, was also recorded (Figure 1).
Calculation of procedure parameters10–12Platelet yield was determined by:
Product volume (mL) × product count (platelets/μL) × conversion factor (1000μL/mL)
Platelet collection efficiency (CE) was calculated by:
Platelet yield / Total platelets processed × 100
Total platelet processed = (pre- + post-count platelets/μl)/2 × total blood volume processed (ml) × conversion factor (1000 μl/mL)
Total blood volume processed = Blood volume processed (ml) - anticoagulant (ml)
Collection Rate (CR) calculated as Yield/procedure time (CR/min)
Recruitment factor (RF) calculated by the formula (Post-donation cell count + yield)/Pre-donation cell count
Platelet loss was derived by Pre-platelet count in whole blood - post donation count/Pre-platelet count in whole blood × 100
The statistical analysis was performed using the Statistical Package for the Social Sciences (SPSSInc, Chicago, IL, Version 22.0 for Windows). All quantitative variables were estimated using measures of central location (mean) measures of dispersion (standard deviation). Qualitative or categorical variables are described as frequency and proportions. To verify the relationship between two variables, the Spearman or Pearson correlation coefficient was calculated. All the statistics were two-sided, performed at a significance level of α = 0.05. A p-value of < 0.05 was considered significant.
ResultsDuring the study period, a total of 108 donors were found eligible for plateletpheresis, out of which 8 were excluded from the study, as consent was not given. Among the 100 donors, 64 were found fit for DDP extraction, but 14 donors were not willing to undergo this procedure, hence, 50 donors were included in the study group, having a platelet count > 250 × 109/L, and the remaining 50 were included in control group, with a platelet count > 150 × 109/L. All the donors were males, out of which 71% were replacement donors and the rest (29%) were voluntary donors. The mean age was 29.24 ± 6.78 years in the control group and 28.70 ± 5.96 years in the study group. Donor baseline characteristics are shown in Table 1. Out of 100 donors, 37 donors had platelet count between 251 and 300 × 109/L, of which 9 (18%) were in the control group and 28 (56%) were in the study group. In the control group, 31 donors had a platelet count of 201 to 250 × 109/L, while in the study group, 43 donors had a platelet count between 251 and 350 × 109/L. Only 4 donors in the study group had a platelet count above 351 × 109/L.
Donor baseline parameters.
The mean pre-donation hemoglobin of donors was 15.34 ± 1.12 g/dL in the control group and 15.00 ± 1.35 g/dL in the study group while the post-donation hemoglobin was 15.12 ± 1.85 g/dL in the control group and 15.02 ± 1.33 g/dL in the test group. The difference was statistically insignificant (p > 0.05). Concerning the hematocrit, there was a decrease of 1.09 (2.48%) in the control group and 0.61 (1.40%) in the study group, which was statistically significant (p < 0.05). The mean pre-donation platelet count of donors was 238.56 ± 35.42 × 109/L (Range 182 – 326 × 109/L) in the control group and 300.40 ± 1.79 × 109/L (Range 248–450 × 109/L) in the study group, while the post donation platelet count was 149.69 ± 45.91 × 109/L (Range 86–222 × 109/L) in the control group and 185.34 ± 35.17 × 109/L (Range 112 – 263 × 109/L) in the test group. The mean decline in the control group was 88.87 × 109/L (37.25%) and in the study group, 115.06 × 109/L (41.63%). The difference in the post-procedure decline in the donor platelet count was statistically significant between the study and control groups (p = 0.000).
A Wilcoxon signed-rank test revealed a statistically significant decline in the post- and pre-hematocrit (Z = −2.602; p = 0.000), platelet count (Z = −6.155; p = 0.000), mean platelet volume and platelet distribution width (PDW) in the control group. In the study group, the difference was significant for the platelet count (p = 0.000), mean platelet volume (p = 0.038) and PDW (p = 0.001) (Table 2).
Wilcoxon signed-rank study for hematological parameters in both groups.
| Group | Hb(g/dl) – Post- procedure - Hb(g/dl) – Pre- procedure | Hct(%) – Post- procedure - Hct(%) – Pre- procedure | Plt(109/l) – Post- procedure - Plt (109/l) – Pre- procedure | MPV(fl) – Post- procedure - MPV(fl) – Pre- procedure | PDW – Post- procedure - PDW – Pre- procedure | |
|---|---|---|---|---|---|---|
| Control Group | Z | −0.378a | −2.602a | −6.155a | −4.299a | −4.118a |
| Asymp. Sig. (2-tailed) | 0.705 | 0.009 | 0.000 | 0.000 | 0.000 | |
| Study Group | Z | −0.648b | −1.752a | −6.155a | −2.072a | −3.371a |
| Asymp. Sig. (2-tailed) | 0.517 | 0.080 | 0.000 | 0.038 | 0.001 | |
The levels of all machine- and procedure-related variables were assessed during and after the procedure. The mean post platelet count of donors calculated by machine was 158.28 ± 37.068 × 109/L in the control group and 189.80 ± 33.300 × 109/L in the study group, while the mean post-hematocrit calculated by machine was 40.57 ± 4.37% in the control group and 40.57 ± 3.480% in the test group. The post-platelet count difference was statistically significant (p = 0.000) between the two groups (Table 3).
Effect of plateletpheresis on machine-related parameters.
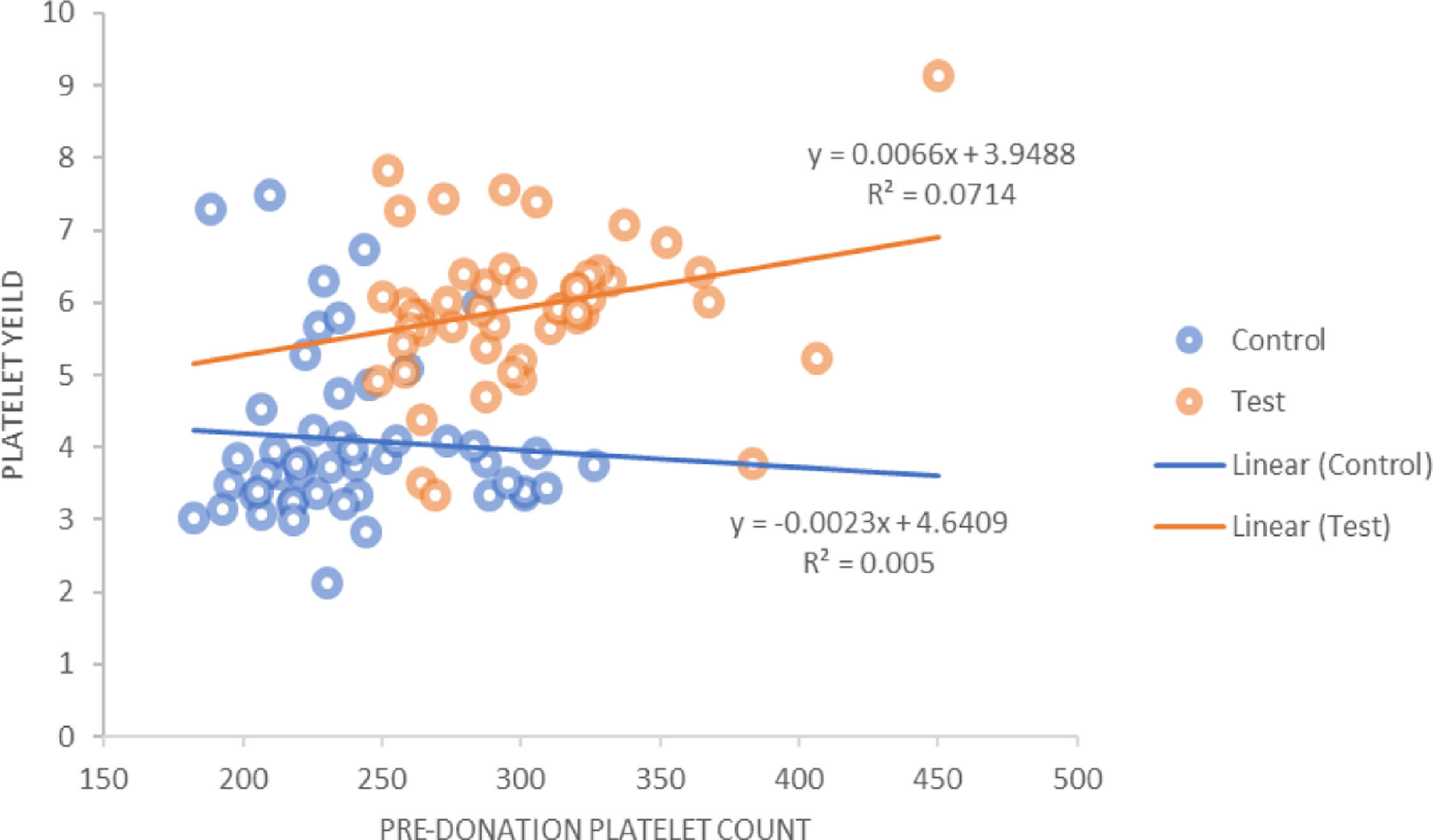
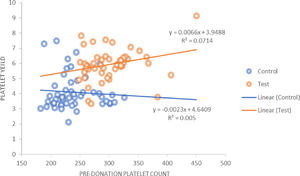
The platelet yield achieved for the control group against a target yield of 4 × 1011 was 4.09 ± 1.15 × 1011, whereas in the study group, in which the target yield was 6 × 1011, the mean yield achieved was 5.93 ± 1.04 × 1011 (p = 0.000). It was also observed that in the control group there was a positive correlation between the hemoglobin, hematocrit and PDW of the donor with the platelet yield. However, in the study group, the hemoglobin, hematocrit and platelet count showed a positive correlation with the platelet yield. For the pre-donation platelet count, there was a statistically significant (r = 0.267; p = 0.000) positive correlation in the study group (Figure 2).
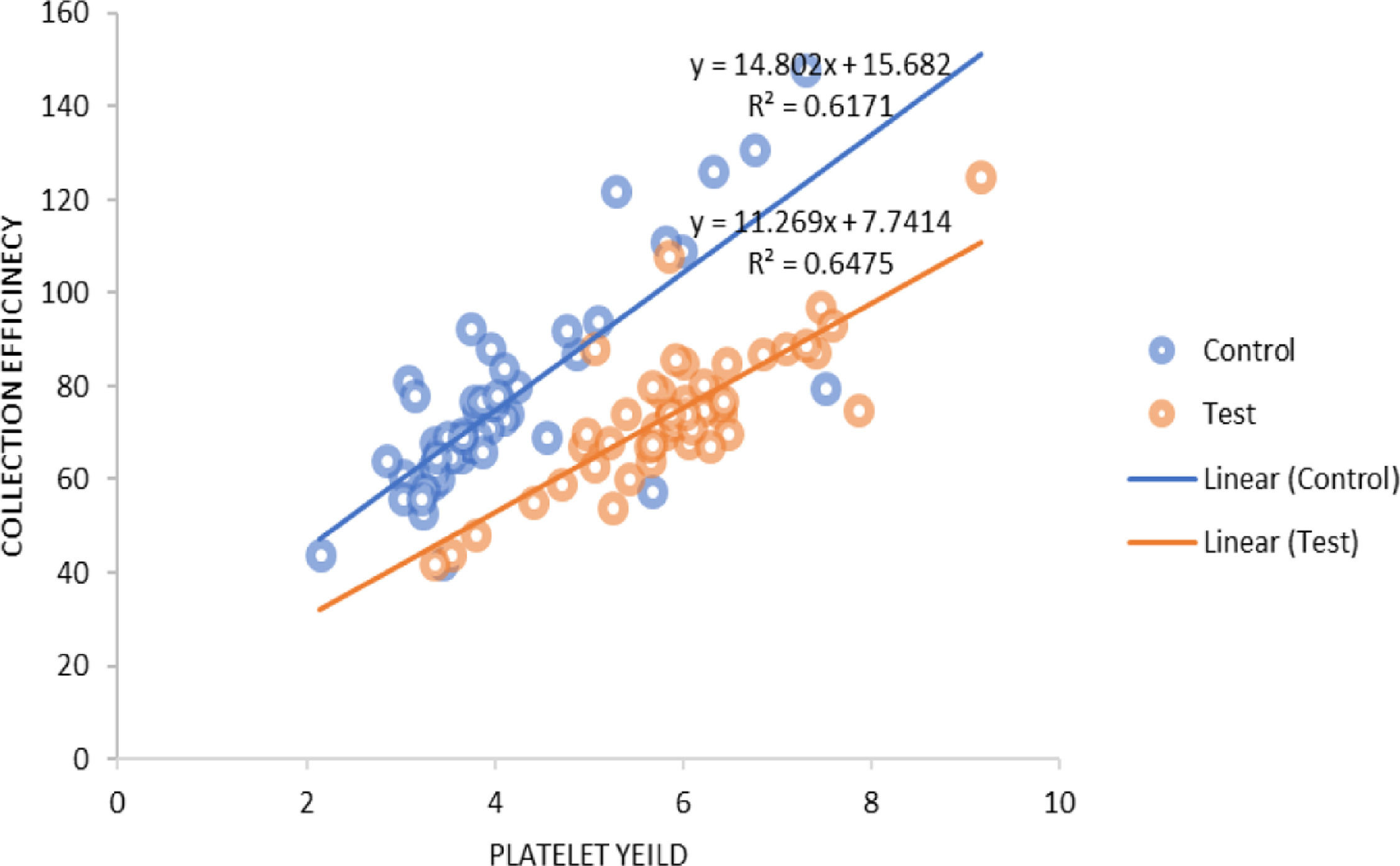
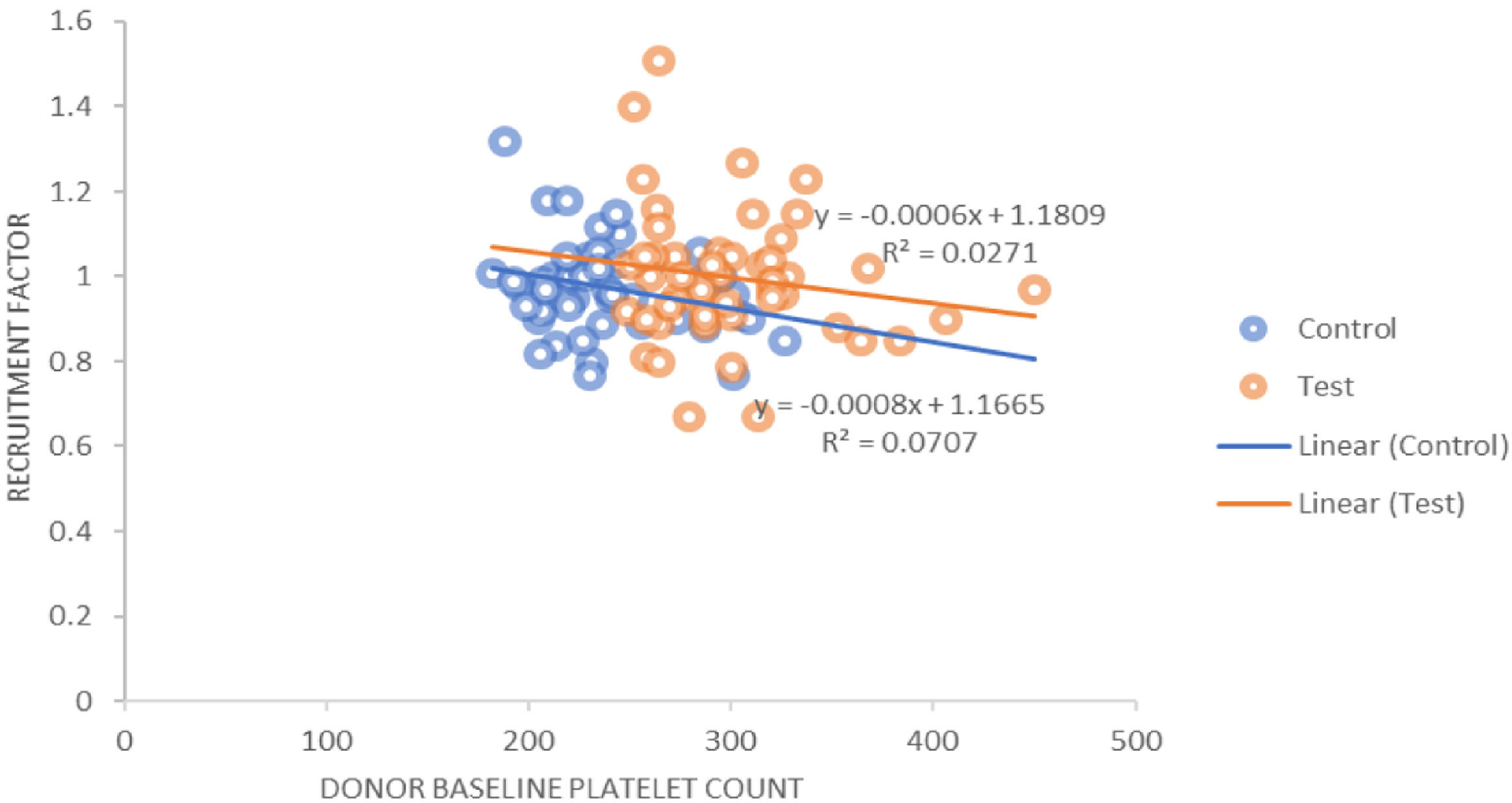
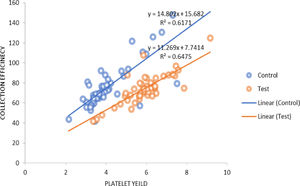
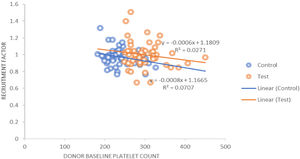
The procedure parameters are summarized in Table 4. In both the study and control groups, volumes of product, total platelets processed and collection rates were positively correlated with the product yields. A similar positive correlation was observed for the collection efficacy (Figure 3) and recruitment factor with the product yield. The platelet loss showed a positive correlation with the yield in the control group, whereas it showed a negative correlation in the study group. In the control group, the recruitment factor was 0.97 ± 0.11, while in study group, the recruitment factor was 0.99 ± 0.16, ranging between 0.67 and 1.51. There was a negative correlation between the donor baseline platelet count and recruitment factor (Figure 4).
Procedure parameters in both groups.
None of the donors in the control group presented adverse reactions, while 3 donors in the study group experienced adverse reactions during the procedure. Two were citrate reactions in the form of paraesthesia, tingling and perioral numbness. One donor developed local reactions in the form of hematoma.
DiscussionPlateletpheresis has proven to be a boon in the field of Transfusion Medicine, as it provides a therapeutically beneficial component and ensures reduced donor exposure.13 Technological advancements have made it possible to collect double, and even triple, doses of platelets amid a shrinking donor population. The platelet concentrates prepared with the help of automated cell separators are of high quality. However, plateletpheresis also has its impact on donor hematological parameters and, with the increasing use of high yield plateletpheresis procedures, donor safety is an area of concern.
Donors included in our study were predominantly males, as in most studies, which also observed that women were associated with complications related to the venepuncture and were at increased risk of vasovagal reactions and adverse events, compared to males.14–16 Tomita et al. found the incidence of vasovagal reactions among female apheresis donors to be 1.25%, while the rate among male donors was 0.83%.17 Studies conducted by some authors considered the body weight for the platelet yield. Donors with weight below 65 kg had a lower platelet yield when DDP collection was performed.18 However, in our study, we did not find any such correlation between weight and height of donors and platelet yield. Another study from India also did not find any significant effect of donor age, weight and height on platelet yield.19
We used a continuous type of cell separator in our study for plateletpheresis donation that is the Amicus, which has an extracorporeal volume of 210 ml. The amount of anticoagulant (ACD) transfused to donors used was 365.09 ± 73.552 ml in the control group, while in the study group it was 443.78 ± 52.430 ml. The mean whole blood volume processed and total time taken were higher in the study group, as expected. In a study to evaluate the Amicus separator in the collection of apheresis, the authors found that the Amicus efficiently collected single, as well as double, apheresis with a mean platelet yield of 4.2 × 1011 and 6.5 × 1011, respectively. All apheresis performed were leukoreduced below 5.0 × 106, while one was below 1.0 × 106.20
Fontana et al. reported a PLT yield of 6.06 × 1011 in double yield collection using the Amicus.21 Similar results were found by Keklik et al.,22 while higher yield of 7.24 ± 0.53 × 1011 has been reported by other authors.23 The DDP yield in our study of 5.93 ± 1.04 × 1011 was comparable to that of another study from India24 but was lower, when compared to studies by other authors.21–23 This could be due to differences in pre-procedure platelet count and target yield in various studies.
Positive correlation between the hemoglobin, hematocrit and pre-donation platelet count of donor and platelet yield was seen in the study group. For the pre-donation platelet count, there was a statistically significant positive correlation in the study group. In the control group, the hemoglobin showed a positive correlation with the platelet yield. The platelet count showed a slight negative correlation in the control group, with respect to the platelet yield. This could be due to procedure-related problems, such as the low flow rate observed in some procedures. Another study also showed a positive correlation between the donor pre-donation platelet count and the platelet yield in DDP and suggested that the donor platelet count is an important determinant for the yield and that a count below 225 × 103/μL is not suitable for DDP collection.18 Authors have suggested that DDP collection may be performed when the donor platelet count is above 250 × 103/μL.25
The relation between the donor baseline platelet count and the yield was analyzed. Out of 50 donors in the study group, 19 had a platelet count in the range of 250 to 280 × 109/L and the mean yield was 5.696 ± 1.57 × 1011 in the product obtained from these donors, while 9 donors had a yield less than 5 × 1011. For 11 donors with a platelet count ranging between 280 and 300 × 109/L, the mean yield was 6.07 ± 0.96 × 1011 and with counts greater than 300 × 109/L, the mean yield was 6.14 ± 0.99 × 1011. The higher the platelet count, the more platelets are available for collection. In our population, donors with a baseline platelet count ≥ 280 × 109/L are more likely to donate double-yield platelet products.
Guerrero-Rivera et al.26 and Enein et al.27 also observed that the pre-donation platelet count was directly proportional to the platelet yield, while an inverse relationship was observed with donor hemoglobin and platelet yield.26 They also observed a significant reduction in the hemoglobin, hematocrit, leucocyte and platelet count of the donor after the procedure.27 Authors from India have also demonstrated the direct relationship between the pre-donation platelet count and platelet yield.12,19 They also suggested that, in the Indian scenario, it would be more relevant to follow the European guidelines of a platelet yield > 2 × 1011 platelets per unit.19
On correlation of machine parameters with the platelet yield, we observed that the ACD used and the apheresis derived platelets showed a positive correlation in the control group and a negative correlation in the study group. The total time taken was negatively correlated in both groups, while flow rate showed a positive correlation. All these parameters were statistically significant between the two groups. Enein et al. analyzed the impact of various donor- and machine-related parameters on the platelet yield in 127 procedures. They found that the anticoagulant infusion rate, total blood volume processed and time taken had a positive impact on the platelet yield.27 Beyan et al. found that factors, such as the maximum draw rate, maximum return rate, whole blood processed, processing time and pre-procedure platelet count could have a significant effect on the yield in plateletpheresis.28
We also observed a decrease in donor hemoglobin in the control group and a mild increase in the study group. The hematocrit showed a decline in both groups which was significant. The increase in the donor Hb in the study group may be because red cells are transfused back to the donor and 300 to 400 ml of plasma is retained at the end of the procedure. Due to this, there is a hemoconcentration in the donor. Tendulkar et al.29 and Das and workers30 found a statistically significant decline in the donor Hb, Hct and platelet count post-procedure, without any clinical evidence of anemia and thrombocytopenia.
The change in the donor platelet count after donation was also analyzed. The decline in the donor platelet counts observed in the control and study groups were 37.25% and 41.63%, respectively, and the difference was statistically significant. A decrease in the mean platelet volume (MPV) and PDW was observed in both groups. Strasser et al.11 reported a platelet count decrease of 27.1 × 103/µl (7.7%) post-donation using the machine AS TEC 204, while a decrease of 26 × 103/µl (12.9%) was reported with the COBE spectra. A higher decline in our study could be attributed to the timing of the post-donation sample and a different cell separator. Das et al., using five different cell separators, observed that there was no significant change in donor MPV or PDW after each procedure in 457 plateletpheresis procedures.30
The total platelet volumes processed in our study were 5.42 ± 1.08 × 10 and 7.95 ± 0.77 × 1011 (p = 0.000) in the control and study groups, respectively. The collection efficiency observed in our study was 71.93 ± 25.14% in the control group and 72.07 ± 16.28% in the study group. Makroo et al.12 reported the CE to be 78.09%, while the CE observed by Chaudhary et al.24 was 59.7% and, in the study by Jaipian et al.,23 85.31%, for DDP collection using the Amicus. In a study conducted on the Trima Accel cell separator, the authors reported efficient DDP collection with a median PLT yield of 3.7 × 1011, mean CE of 74.99 ± 14.40% and mean CR of 0.096 ± 0.012 × 1011/min.31 The collection rate found in our study was 0.78 ± 0.12 × 1011/min. and 0.94 ± 0.13 × 1011/min. in the control and study groups, respectively.
The recruitment factor was 0.97 ± 0.11 in the control group, while in the DDP group the recruitment factor was 0.99 ± 0.16, ranging between 0.67 and 1.5 (p = 0.313). We also observed the platelet loss of 35.55 ± 8.53% in the control group, while in the study group it was 37.76 ± 8.65 (p = 0.200). Fontana et al.21 found that the PLT recruitment (1.56 ± 0.31) caused a higher post-PLT count than that predicted by the instrument (p < 0.0001).
In both groups, the product volume, total platelets processed, collection rate, collection efficacy and recruitment factor were found to be positively correlated with the product yield. The platelet loss was positively correlated with the yield in the control group, while it was negatively correlated in the study group. The product volume and total platelet product were found to be statistically significant (p = 0.000). Strasser et al. found a negative correlation between the baseline platelet count and recruitment factor (r = −0.38; p = 0.003), while there was a positive correlation between the platelet yield and recruitment factor (r = 0.44; p = 0.001), as well as between the collection efficacy and recruitment factor (r = 0.025; p = 0.05). They also studied the correlation of different variables which could affect the platelet yield and collection efficacy and found a strong negative correlation between the platelet baseline counts and RF (r = −0.662; p < 0.001).11
Plateletpheresis procedures are well tolerated, but sometimes donors may experience adverse reactions that can be localized or systemic. In our study, out of a total of 100 donors, no donors in the control group showed any adverse reactions, while 2 donors in the study group experienced citrate reactions, which were corrected by oral calcium supplementation, and one had a local reaction. Chaudhary et al.24 found adverse effects in 22.4% of their donors who underwent DDP collection. In a recent retrospective study, the adverse reaction rates were reported at 10.34% in high-yield procedures and 5.68% in normal-yield procedures. The authors reported 3.23% of phlebotomy-related complications and 2.28% of reactions due to citrate toxicity.32
We also assessed the quality of apheresis platelet concentrates. Swirling was present in 93% of the units. The platelet count was over 3 × 1011 in all fifty units in the control group and over 6 × 1011 in twenty-three units in the study group. The individual yield in split products in the study group was 2.74 ± 0.55 × 1011. The authors reported that the fresh PCs have swirling in 83% of the units, which was reduced to 65% after 5 days of storage. They attributed this decline to storage lesions that are known to occur after platelet preservation.33 The AABB requires that 95% of the units tested have a leucocyte count ≤ 5 × 106. In our study, the average leucocyte count in products from the control group was 2.88 ± 0.2 × 106 and in the study group, 1.64 ± 0.5 × 106; hence, all products were leukoreduced.
There were certain limitations in our study. All donors included in the study were males, so the effect of plateletpheresis on female donors could not be studied. The donor sample was obtained soon after the completion of the procedure and hence, the change in hematological parameters observed may not be representative. It would have been desirable to have a daily hematological parameter post-donation assessment to determine the trend of the platelet recovery, but due to logistic problems and the convenience of the donors, this could not be performed. Although no significant adverse events were observed in donors undergoing DDP, the frequency of high-yield procedures and its effect on donor safety were not studied in our population due to the limited donor pool in this subset. However, we conducted a comprehensive analysis of data comparing single- and double-dose plateletpheresis which would encourage transfusion medicine specialists to employ double-dose platelet collection among eligible blood donors in developing countries, which will go a long way in reducing cost and, at the same time, the transfusion risks.
ConclusionWe conclude that double-dose or higher yield plateletpheresis can supplement limited platelet inventory and limit donor exposure, which can further be of immense importance in the setting of a developing country. Donors with lower MPV and PDW tend to donate products with a higher yield. Donors with a higher platelet count yield DDP products with higher yield. With improved automated platforms, procedure duration is shorter, even in high-yield products, thus enhancing donor comfort.
Ethical approval statementThe study was approved by the institutional ethics committee.
Donor consent statementAll donors who participated in the study gave written informed consent.
The study did not receive funding from any agency.