Diversity in Classical Hematology Research
More infoThe remission induction treatment for acute myeloid leukemia (AML) has remained unchanged in the resource-limited setting in the Philippines. AML treatment consists of induction chemotherapy followed by high dose consolidation chemotherapy or allogeneic hematopoietic stem cell transplantation. In the Philippines, the Filipino household bears the burden of health care cost of hospitalization expenditure. Insights into the treatment costs becomes an essential requirement as these guides the allocation of resources to scheme health programs.
MethodThis study involved a retrospective cohort analysis of AML patients who underwent treatment for AML. Review of the statements of account per admission per patient during treatment for remission induction, consolidation, relapsed and refractory disease and best supportive care from 2017 to 2019. Of the 251 eligible patients, 190 patients were included.
ResultThe mean healthcare expenditure for remission induction chemotherapy (Phase 1) was US $2, 504.78 (Php 125,239.29). While 3 to 4 cycles of consolidation chemotherapy cost an average of US $3,222.72 (Php 162,103.20). For patients who had relapsed and refractory disease, an additional mean cost of US $3,163.32 (Php 159,115.28) and US $2, 914.72 (Php 146,610.55) were incurred, respectively. The average cost of palliative care was US $1,687.00 (Php 84,856.59).
ConclusionThe cost of chemotherapy and other therapeutics bear most of the weight of the direct healthcare cost. The cost of AML treatment represents a significant economic burden for patients and the institution. The cost increases as patients proceed through subsequent lines of treatment for induction failure. Existing subsidy for health insurance benefits could still be improved for appropriate source allocation of resources.
The remission induction treatment for acute myeloid leukemia (AML) is intensive and has remained unchanged in the resource-limited setting in the Philippines. The AML treatment consist of induction chemotherapy followed by high-dose consolidation chemotherapy or allogeneic hematopoietic stem cell transplantation.1 In-hospital costs of induction are the most expensive, while pre-existing co-morbidities and complexity of cases requiring intensive care unit (ICU) admissions and management of complications are contributory. When comparing the costs among the different phases of AML treatment, multiple factors play a role in the analysis of estimates. These include funding models, nursing models, healthcare system, universal health insurance coverage and best available treatment.
Healthcare is not optimized for most of the marginalized population, outcomes have ceased to improve because of the prohibitive costs of the allogeneic hematopoietic stem cell transplantation (HSCT). According to Baylon et al., one of the major challenges in sustaining a bone marrow transplantation program in the Philippines is the cost of medical care.2 Insights into the treatment costs becomes an essential requirement, as the health-seeking behavior of patients and outcomes measures.3
Out-of-pocket spending for catastrophic diseases remains high and financial protection remains low. Succeeding costs will be out- of- pocket expenses unless the patient is admitted to the service wards, in which the no-balance billing scheme can shoulder the cost of the admission.4 The data regarding the economic burden of AML this study aims to provide can help guide the allocation of resources to plan health programs that are cost-effective, ameliorating health insurance coverage for Filipinos with AML. Cost evaluation and understanding can re-organize our approach to improve healthcare provisions as physicians and, hopefully, contribute to the long-term survival and quality of life of our patients.
The Filipino household bears the burden of the healthcare cost of hospitalization and expenditure.5 There have been no local studies yet that have calculated the cost of the AML treatment in a resource-limited setting. The aim of the study was to determine the direct cost of the AML treatment in a tertiary hospital in the Philippines.
MethodsPopulationThe study was a retrospective cohort analysis of all AML patients with confirmed diagnosis by bone marrow aspirate or peripheral blood flow cytometry who received initial treatment for AML at the Philippine General Hospital (PGH) from 2017 to 2019.
The population included all adult (age ≥ 19) patients diagnosed with AML from 2017 to 2019. Their course was followed from the time of the initiation of the inpatient treatment until 1 year later, or until death or loss to follow-up, whichever came first. The retrieval and evaluation of records of each hospitalization or confinement for each patient were performed. This included ICU, ward and emergency room confinements.
Inclusion criteria:
All patients diagnosed with AML who were
- •
≥ 19 years of age at the time of diagnosis
- •
admitted for initial treatment of AML at PGH from January 2017 to December 2019
Exclusion criteria:
- •
Clonally evolved AML, chronic myeloid leukemia (CML) in blast crisis, treatment-related AML, AML with concomitant malignancy
- •
Patients who opted to undergo treatment with hypo-methylating agents as first-line therapy
- •
For patients who had undergone HSCT at another hospital, the cost of the allogeneic hematopoietic transplant will not be included
Direct healthcare costs of hospitalization for the treatment of AML were included. The cost was retrieved from each unique admission for one particular patient. Each admission was included and stratified into 5 phases: Phase 1 – remission induction regimen; Phase 2 - consolidation regimen; Phase 3 – relapsed cases; Phase 4 –or refractory cases, and; Phase 5 – Best Supportive Therapy.6 Direct healthcare costs included the charges incurred by the patient during each inpatient hospitalization, as reflected in the statement of account.
Direct non-healthcare costs and indirect costs of healthcare, such as productivity losses, job absenteeism, limb impairment, foregone leisure time and the time spent by visitors and family attending to patients were not included. Data on outpatient visits or hospitalizations at institutions other than PGH were not included. Likewise, the physician service fee and reader's fee were not included for the private inpatient cases. The cost of establishing the diagnosis of AML, if incurred as an inpatient, was not included.
The estimation of the lifetime cost of the condition for this study utilized the incidence-based method. It focused on the direct healthcare cost from the hospital standpoint. The bill incurred for each patient hospitalization from the initial treatment until 1 year later, death, or loss to follow-up were retrieved through the credit and billing department. The sum of the total cost of confinements was tallied. Diagnostic procedures and management incurred as an outpatient were not included to keep the results homogenous and conclusive. Payments made outside of the PGH system while the patient was admitted were not included. All treatment modalities that were performed and billed to the patient as an inpatient case were included. For medicines acquired during the hospitalization through the Philippine Charity Sweepstakes Office (PCSO) or other non-governmental organization (NGO), the estimated cost of these medicines was included based on the institutional cost per medicine utilized.
The cost analysis was distinguished based on five phases; namely, Phase 1 – remission induction regimen; Phase 2 - consolidation regimen; Phase 3 – relapsed cases; Phase 4 –or refractory cases, and; Phase 5 – best supportive therapy. If patients with refractory disease achieved remission after re-induction, the costs of all succeeding treatments were classified under refractory cases (Phase 4). Likewise, for relapsed cases which achieved a second remission after re-induction, all costs after this treatment were classified under relapsed cases (Phase 3). Total costs of all treatment-related hospitalizations, supportive therapy and management of complications until remission or death were summated. These were evaluated and computed based on the value of the Philippine peso (Php) and rounded up to the nearest centavo (cent). The costs of each admission were subclassified to blood bank and transfusion services, diagnostics, chemotherapy and other therapeutics, and room and board.
Statistical analysisThe estimate of the direct cost was obtained using the bottom-up approach.7 This was the option utilized to provide an unbiased estimate of the cost of the disease, avoid confounding variables and average the cost of illness per phase set by the investigator. Only the direct costs were taken into consideration. Although equally important, the indirect cost incurred was not included, as no patient interaction was sought to complete the data. No missing data was replaced or estimated.
Accrued costs were reported based on the phases. Sensitivity analysis was utilized to allow for uncertainties and verify the robustness of the variables for each phase. Regression analysis was utilized to account for confounding variables. Cost-to-charge ratios were applied as necessary to reflect true cost of the service.
In this study, the number of hospital days and cost of hospital services rendered were described using average, standard deviation and CI at 95%. The cost-to-charge ratio is the ratio between hospital expenses and what they charge. The closer the cost-to-charge ratio is to 1, the less difference there is between the actual costs incurred and the hospital charges. For service patients, the cost-to-charge ratio was zero, as the no-balance-billing scheme were observed. The cost-to-charge ratio, in theory, for private/pay patients is close to one. All patients, service and private, pay for most diagnostics and therapeutics on an out-of-pocket basis. The bulk of the hospital subsidy for charity patients is the free ward accommodation. Private/Pay patients pay for the full cost of their board.
Comparisons of cost-to-charge ratios among phases of AML Illness and weighing costs of the AML phases were obtained using the Multifactorial ANOVA if the phases were independent, while the Repeated Measures ANOVA was used when comparisons of paired or repeated phases are analyzed. Moreover, a General Linear Model (GLM) repeated measure regression analysis was utilized to account for confounding variables associated with cost, cost-to-charge ratios and weighing costs of AML. Subsequently, finalizing model fit and testing assumptions were made to ensure that the regression model is valid. The final regression model will estimate cost, cost-to-charge ratios and weighing costs of AML, as influenced by significant predictors. Any associated p-values under 0.05 alpha were considered significant.
Categorical variables were presented as frequencies and proportions, while continuous variables were summarized by providing the mean, median and standard deviation (SD). The data was tabulated and analyzed using the Google Sheets with the aid of the XL Miner Analysis Toolpak.
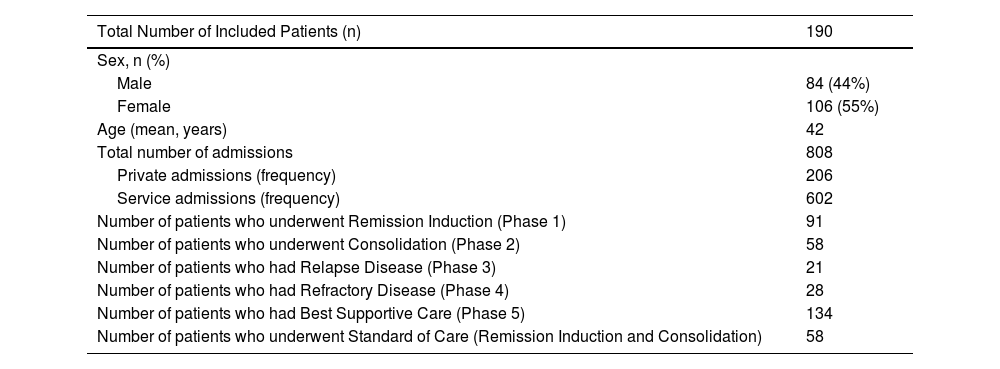
ResultsA total of 251 patients were included in the study from January 2017 to December 2019,8 from which 190 patients with AML who underwent treatment were included in this analysis. The mean age of the patients included in the study was 42 years old. This reflects the mean age group, which was offered the standard treatment, remission induction chemotherapy. There were a total of 808 admissions for AML treatment (Table 1). Each patient was admitted on an average of 4.25 times for the entire course of the treatment. Patients had an average hospital stay duration of 63.78 days for the entire treatment course, encompassing all phases of treatment.
Demographic profile and Clinical Characteristics.
| Total Number of Included Patients (n) | 190 |
|---|---|
| Sex, n (%) | |
| Male | 84 (44%) |
| Female | 106 (55%) |
| Age (mean, years) | 42 |
| Total number of admissions | 808 |
| Private admissions (frequency) | 206 |
| Service admissions (frequency) | 602 |
| Number of patients who underwent Remission Induction (Phase 1) | 91 |
| Number of patients who underwent Consolidation (Phase 2) | 58 |
| Number of patients who had Relapse Disease (Phase 3) | 21 |
| Number of patients who had Refractory Disease (Phase 4) | 28 |
| Number of patients who had Best Supportive Care (Phase 5) | 134 |
| Number of patients who underwent Standard of Care (Remission Induction and Consolidation) | 58 |
The entire course of treatment had a mean cost of US$ 4225.28 (Php 212,532) per patient. Each admission cost each patient an average of US$ 993.63 (Php 49,979.72), regardless of the treatment phase. The hospital subsidy for charity patients is on average US$ 568.18 (Php 28,579.71) per admission for room and board. For private cases, cost-to-charge is assumed at 1 since the actual cost is the exact amount charged. The cost-to-charge ratio for charity patients relative to the baseline is computed at 0.64. Private patients spent an additional US$ 1197.50 (Php 60,234.42) for the room and board for the entire course of their treatment.
Not all patients undergo the entire phase of their chemotherapy at the same institution. For patients who were admitted from another hospital for the continuation of chemotherapy, their admission is included depending on whether they are in consolidation (Phase 2), reinduction (Phase 3 or 4), or best supportive care (Phase 5). Some admissions that are included had not necessarily started from phase 1 and not all patients included in that phase were necessarily relapsed nor refractory.
Not all patients admitted started as chemotherapy naïve, which would make them fall under phase 1. At some point in a particular patient's life, he or she would be admitted to the institution as having refractory/relapsed disease and, hence, the cost of treatment during that phase was included in Phase 3 or 4.
The Best Supportive Care6 patients are those admitted for blood transfusion, IV fluid hydration and treatment of complications during chemotherapy. No chemotherapeutic agents were given during this time. Patients between cycles who had been admitted for pneumonia, for example, would be admitted under the best supportive care, as these would be included for the management of complications from AML treatment and not necessarily from chemotherapy.
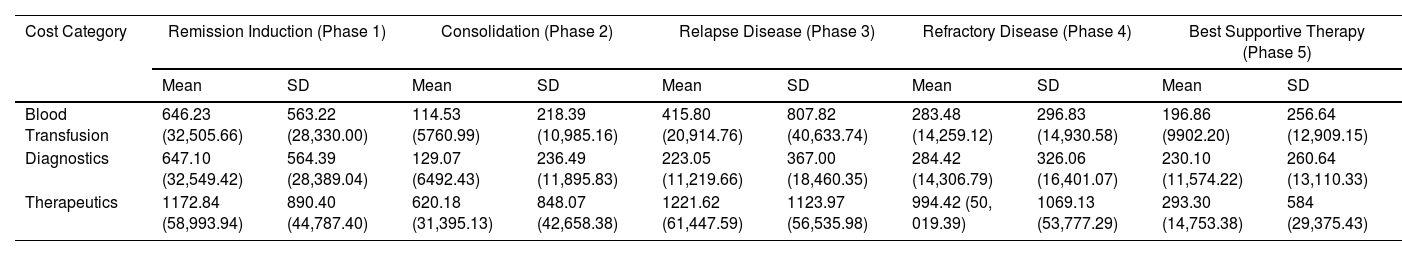
Among the 190 patients, 91 patients underwent the remission induction chemotherapy (Phase 1) (Table 1). The average cost of chemotherapy and other therapeutics for this phase was US$ 1172.84 (Php 58,993.94, exclusive of diagnostics, blood transfusion and blood bank services and room and board for service patients) (Table 2).
Distribution of direct costs (Average) incurred during AML treatment per admission US$ (and Philippine Peso) (in 2017- 2019, 1 US$ = Php 50.3).19
| Cost Category | Remission Induction (Phase 1) | Consolidation (Phase 2) | Relapse Disease (Phase 3) | Refractory Disease (Phase 4) | Best Supportive Therapy (Phase 5) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Blood Transfusion | 646.23 (32,505.66) | 563.22 (28,330.00) | 114.53 (5760.99) | 218.39 (10,985.16) | 415.80 (20,914.76) | 807.82 (40,633.74) | 283.48 (14,259.12) | 296.83 (14,930.58) | 196.86 (9902.20) | 256.64 (12,909.15) |
| Diagnostics | 647.10 (32,549.42) | 564.39 (28,389.04) | 129.07 (6492.43) | 236.49 (11,895.83) | 223.05 (11,219.66) | 367.00 (18,460.35) | 284.42 (14,306.79) | 326.06 (16,401.07) | 230.10 (11,574.22) | 260.64 (13,110.33) |
| Therapeutics | 1172.84 (58,993.94) | 890.40 (44,787.40) | 620.18 (31,395.13) | 848.07 (42,658.38) | 1221.62 (61,447.59) | 1123.97 (56,535.98) | 994.42 (50, 019.39) | 1069.13 (53,777.29) | 293.30 (14,753.38) | 584 (29,375.43) |
There were a total of 164 admissions under phase 2, consolidation chemotherapy.
For chemotherapy and other therapeutics, each consolidation chemotherapy cost an average of US$ 624.15 (Php 31,395.13) per admission (n = 164). For patients who completed 3 to 4 cycles of consolidation chemotherapy (n = 58), the average cost of consolidation (Phase 2) was US$ 1827.89 (Php 91,942.87). On average, each patient completed 2.93 cycles. The cost of condensed HIDAC (Days 1 - 3) cost each patient US$ 584.66 (Php 29,408.55) per admission. On the other hand, admissions following the regular HIDAC schedule (Days 1, 3, and 5) cost the patient US$ 534.57 (Php 26,889.27), on average.
Each admission under Phase 3 for relapsed cases had an average cost of US$ 1221.62 (Php 61,447.59). These included admissions for chemotherapy and other therapeutics (IV fluids, antibiotics, enteral feeding and use of mechanical ventilator). Each patient had an average cost of US$ 1908.78 (Php 96,011.86), in addition to what was previously spent for remission induction and consolidation. The mean additional cost for Phase 4, refractory disease among the 28 patients analyzed, was an additional average of US$ 994.42 (Php 50,019. 39) per admission. The analysis showed an average additional cost of US$ 1839.67 (Php 92,535.86) in addition to the cost of remission induction and consolidation chemotherapy.
For those who underwent an entire course of standard of care (remission induction and 3 to 4 consolidation chemotherapy cycles), the total cost for the AML treatment was US$ 6729.63 (Php 338,500.40), on average. This was the cost for the service patients, while an additional US$ 1197.50 (Php 60,234.42) was incurred for patients who underwent chemotherapy under the pay services, on average.
The breakdown of the costs was divided as follows, reflected in the billing statements per admission, namely diagnostics, blood transfusion and blood bank services, chemotherapy and other therapeutics and room and board. The chemotherapy weighs heavily on the cost for the phase 1 treatment, as well as the other therapeutics to support the effects of cytopenia following the induction chemotherapy regimen.
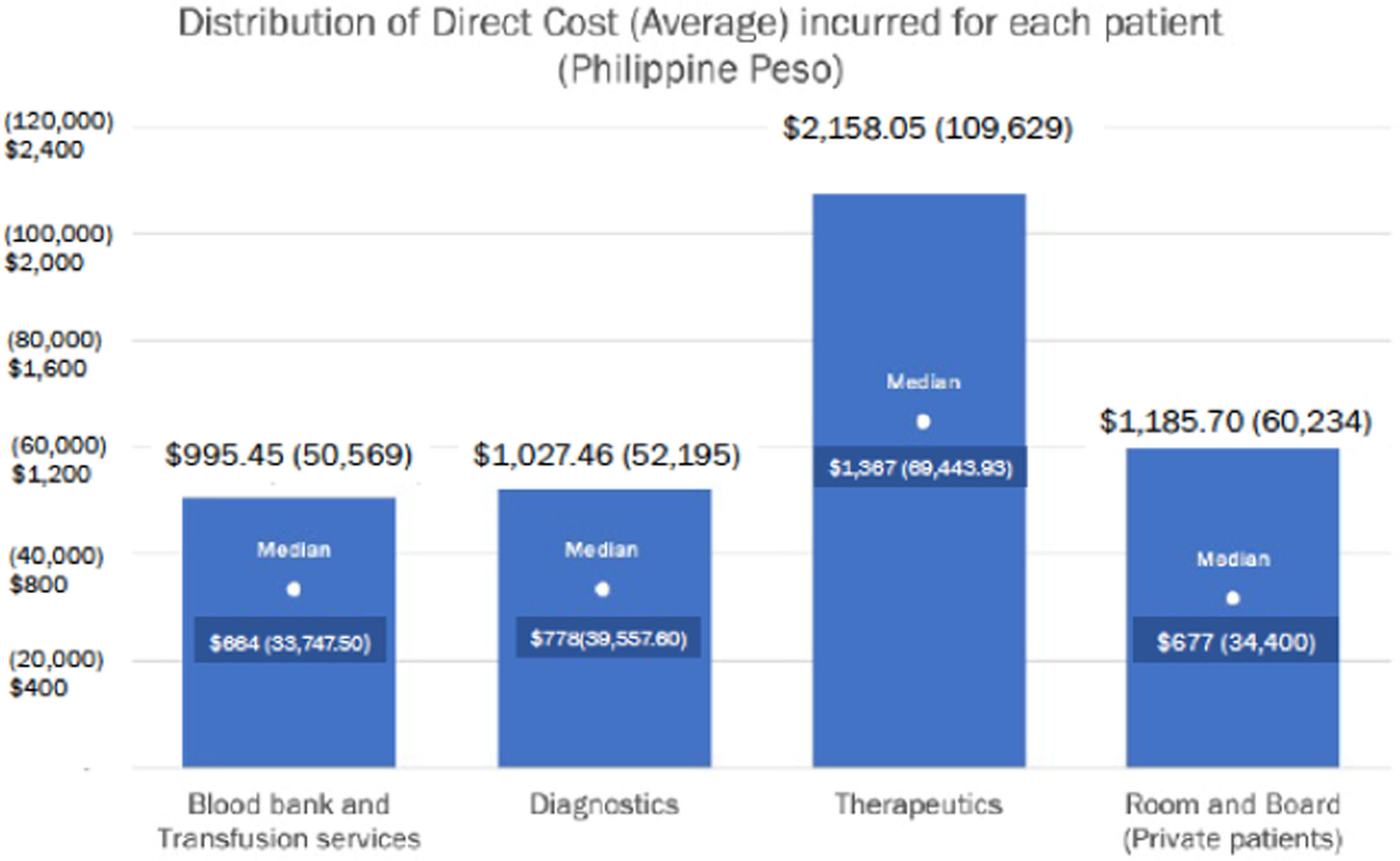
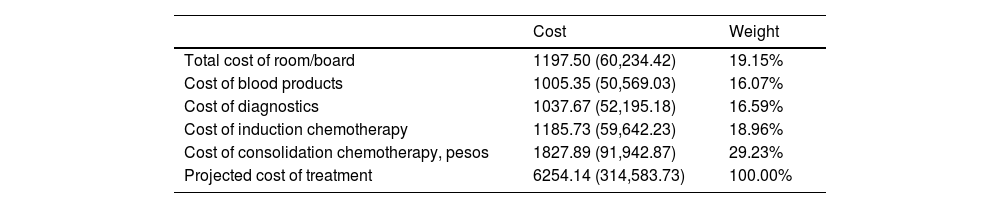
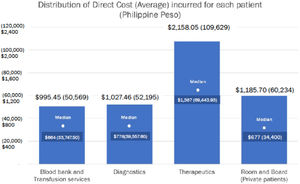
In Table 3, the average weighted costs of room and board, diagnostics, blood bank and transfusion services, induction chemotherapy and consolidation chemotherapy for private patients are presented. The total average weighted costs amounted to US$ 6254.14 (Php 314,583.73).
Average weighted costs of AML treatment for private/pay patients in PGH US$ (Philippine Peso) (in 2017- 2019, 1 US$ = Php 50.3).19
| Cost | Weight | |
|---|---|---|
| Total cost of room/board | 1197.50 (60,234.42) | 19.15% |
| Cost of blood products | 1005.35 (50,569.03) | 16.07% |
| Cost of diagnostics | 1037.67 (52,195.18) | 16.59% |
| Cost of induction chemotherapy | 1185.73 (59,642.23) | 18.96% |
| Cost of consolidation chemotherapy, pesos | 1827.89 (91,942.87) | 29.23% |
| Projected cost of treatment | 6254.14 (314,583.73) | 100.00% |
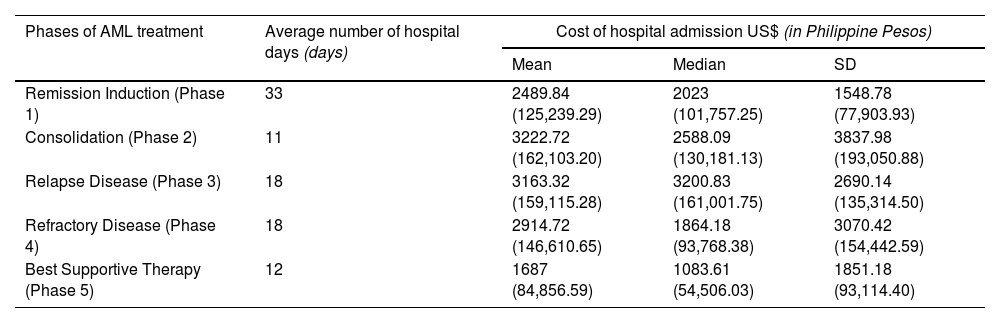
Each phase of AML treatment includes the total cost of the following: diagnostics, ancillaries, blood bank and transfusion services and chemotherapy and other therapeutics. Increasing costs of treatment were observed for patients with refractory disease and those who had relapsed (Table 4).
Average number of hospital days and costs per patient of each phase of AML illness.
| Phases of AML treatment | Average number of hospital days (days) | Cost of hospital admission US$ (in Philippine Pesos) | ||
|---|---|---|---|---|
| Mean | Median | SD | ||
| Remission Induction (Phase 1) | 33 | 2489.84 (125,239.29) | 2023 (101,757.25) | 1548.78 (77,903.93) |
| Consolidation (Phase 2) | 11 | 3222.72 (162,103.20) | 2588.09 (130,181.13) | 3837.98 (193,050.88) |
| Relapse Disease (Phase 3) | 18 | 3163.32 (159,115.28) | 3200.83 (161,001.75) | 2690.14 (135,314.50) |
| Refractory Disease (Phase 4) | 18 | 2914.72 (146,610.65) | 1864.18 (93,768.38) | 3070.42 (154,442.59) |
| Best Supportive Therapy (Phase 5) | 12 | 1687 (84,856.59) | 1083.61 (54,506.03) | 1851.18 (93,114.40) |
The bulk of the burden relies on the therapeutics throughout a patient's course of treatment. Room and board for charity or service patients is zero (Figure 1).
The different tables were each given emphasis to provide data on the costs per phase; average distributive cost of each admission per phase; average weighing cost, as to which of these costs more; average cost per phase, and; the cost incurred by each patient, on average.
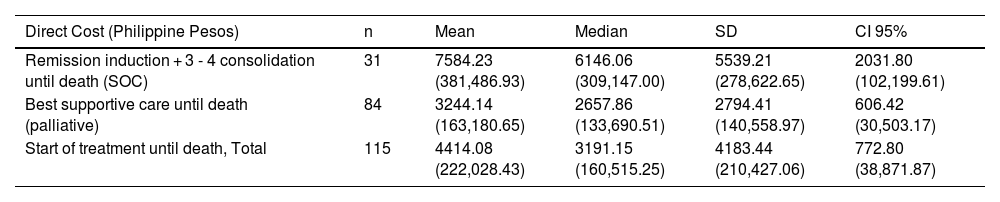
For all the patients who were followed up until death (n = 115), the average cost or treatment was US$ 4414.08 (Php 222,028.43). Of these patients, 31 underwent the standard of care and their average treatment cost was US$ 7584.23 (Php 381.486.93). The remaining (n = 84), who received only palliative care, spent an average of US$ 3244.14 (Php 163,180.65) for treatment.
Among all AML patients included in the study, the average survival was 6.35 months. Among the AML patients who died, but underwent the standard of care, the average survival was 12.87 months. While among patients who died, but only underwent palliative treatment from the definitive diagnosis of AML until death, the survival was 4.02 months (Appendix A).
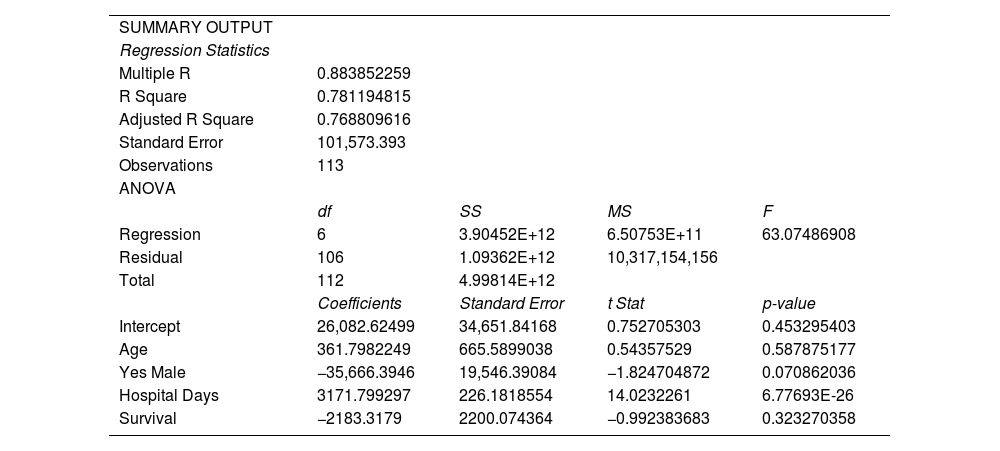
Linear regression statistics were obtained using ANOVA to assess the correlation of total cost with age, sex, survival time and hospital days. The positive value of the coefficient reflects direct correlation to each of the following factors. For the age factor, the older the patient was, there was a positive correlation with the cost of treatment. For the male sex, the total cost of treatment decreased, translating to, and associated with, a US$ 709.07 (Php 35, 666.39) decrease in cost. For patients who underwent the standard of care, it was associated with a higher cost. Lastly, the longer a patient stayed in the hospital, the higher the cost of treatment. However, in summary, as all the p-values for each of the factors is higher than 0. 05, none of the following factors considered were clinically significant. The general linear model was not derived, as there were fewer than 5 variables observed Appendix B.
Discussion and conclusionThe description of the Philhealth reimbursement health system was placed as Appendix C. The admissions included in this study were patients with no private health insurance to cover their treatment cost. None of these patients were admitted under health maintenance organizations or HMOs, in which prepaid coverage of treatment is shouldered by annual payment by patients. The discussion and conclusion were made extensive to reiterate that the economic burden of the AML treatment in the Philippines is much heavier than that of other countries, in which universal health care and insurance are available to citizens.
This study provides insight into the current cost of the initial treatment of AML in a resource-limited tertiary hospital in the Philippines. It shows that the economic burden of the treatment costs of AML is significant to both institution and patients. Compared to highly developed countries (United States), the cost is 86% lower than the previously estimated treatment cost.9 The driver for the high cost is the number of hospital days and treatment.
Compared to the first-world population, the cost of the AML treatment in a third-world country, such as the Philippines, is only 12% of the average cost of the AML treatment.10 It is important to note that the costs reflected in this study are the costs charged to the hospital for the AML treatment. Despite the new frontline recommendations for the AML treatment, access to these options is not easily available. Meanwhile, for patients in the Philippines who can avail themselves of this, the cost is charged as an out-of-pocket expense. Compared to other countries, the cost is cheaper at our institution, as the best treatment available to these patients remains the 3 + 7 regimen, Anthracycline and Cytarabine, with limited access to Idarubicin, Daunorubicin, and other novel agents, are recommended. In addition, provision for bone marrow and stem cell transplantation for patients who have high-risk disease at the baseline are not available at the institution. The data provided by this study covers the cost of the AML treatment from the institutional point of view, as the Philippine General Hospital is a level 3 institution, in which no balance billing is being applied, hence, providing a good estimate measure on the economic burden of the cost of treatment to the patient.
Based on the data collected and analyzed, for each AML patient, there is an average of 4 admissions for the entire course of chemotherapy treatment, with an average stay duration of 63 days. This is exclusive of admissions for blood transfusions.
For the entire course of the chemotherapy, the patients will only be able to avail themselves of a total of US$ 573.22 (Php 29,120) allowable Philhealth benefit (Php 7280 × 4 average admissions for chemotherapy). When compared to the total cost of the entire chemotherapy treatment course per patient, $6663.39 (Php 338, 500.40), the Philhealth allowance benefit only covers 8.6% of the total cost per patient under the standard of care.1112
In the US, the estimated cost of induction chemotherapy ranges from $92,929 to $153,986 (Php 4878,772.50 to Php 8084,265, based on the 2019 USD to Php exchange rate), compared to our institution, each induction chemotherapy (Phase 1) had a mean cost of $2465.34 (Php 125,239.29). Compared to the literature review in 2014, the cost of 1 induction cycle of chemotherapy in 4 countries, the cost of 1 induction cycle in the Philippines is only 6.2% to 22.6% of the cost in Italy, France, England and the United States of America.1314 In the Netherlands, the weighted average cost of the AML induction depends on the regimen available.913
On the other hand, for the consolidation chemotherapy, in the US, the average cost ranges between $29,842 and $70,925 per cycle (Php 1566,705 to Php 3723,562.50, based on the 2019 USD to Php exchange rate). Based on our findings, the average cost of admission for consolidation chemotherapy (Phase II) is $3191.00 (Php 162, 103. 20). This figure is only The figure is only between 4.3% and 10.3% of the total amount this phase costs in the USA.. Based on the regimen used, in the US, the consolidation chemotherapy ranges from $16,119 and $17,120 for the European organisation for Research and Treatment of Cancer (EORTC) and the Haemato-Oncology Foundation for Adults in the Netherlands (HOVON) protocols, respectively, (Php 422, 478 to Php 448,715.2 based on the 1995 USD to Php exchange rate)9, while Hassan, et al. reported that the mean total cost of consolidation chemotherapy (High Dose Cytarabine, HiDAC) is equivalent to $9000 in Egypt (Php 393, 120, based on the 2012 USD to Php exchange rate).15 Findings in this study showed that the cost for the HIDAC 1,2,3 and HIDAC 1,3,5 was $578.90 (Php 29,408.55; n = 87 admissions) and $529.31 (Php 26, 889.27; n = 72), respectively. However, this is only the cost of chemotherapy and other therapeutics under the consolidation (Phase II) treatment. The disparity in cost can be attributed to the other therapeutics used for a particular patient.
Studies have shown10,14,16,17 that the average duration of the hospital stay is a substantial driver of the hospital costs. In our findings, the average duration of the hospital stay for a patient undergoing chemotherapy is 63 days. The length of the hospital stay was s a substantial driver in determining the cost. This is most apparent in the private cases, for which a substantial amount of room and board contributes to the total cost of admission. Additionally, the concurrent additive costs of treatment/therapeutics during the hospital stay adds to the total cost of admission.
The cost of treatment for relapsed AML differs because it is individualized, based on the protocol utilized by the institution for a particular patient. For the patients who were refractory to treatment and those who had relapsed disease, an additional cost of $2886.03 and $ 3132.19 (Php 146,610.65 and Php 159,115.28) were incurred, respectively, being the average cost of admission for relapse or refractory treatment with chemotherapy. The cost is 88 - 90% lower than the cost of relapse and refractory treatment in first-world countries (US, Europe).13,18
The best supportive care option was available for patients who did not consent to chemotherapy. For patients who underwent palliative treatment alone, the average cost per patient was $3212.21 (Php 163,180.65) Comparing the cost of the AML treatment with standard of care chemotherapy with 3 + 7, followed by consolidation treatment after remission induction, the survival of the patients who chose palliative chemotherapy from the time of diagnosis was 4 months, while the survival of patients who underwent the standard of care was 12.87 months. From the survival data in relation to the cost of treatment from follow-up to death, analysis showed that for all AML patients who died, but underwent the standard of care, an additional cost of $4297.36 (Php 218, 306. 28) extended their life for an additional 8 months, compared to those treated with palliative care alone (Table 5). This is 32% less than the cost of best supportive care among highly developed countries. This reflected that, for patients who underwent chemotherapy, the cost of supportive medications for cytopenia and infections contributed to the majority of the total cost of treatment. While it incurred a higher cost, the disease-free survival is extended when definitive treatment is administered, compared to when only supportive medications are given for palliation.
Cumulative direct cost of patient from initial treatment and death in US$ (and Philippine Pesos).
| Direct Cost (Philippine Pesos) | n | Mean | Median | SD | CI 95% |
|---|---|---|---|---|---|
| Remission induction + 3 - 4 consolidation until death (SOC) | 31 | 7584.23 (381,486.93) | 6146.06 (309,147.00) | 5539.21 (278,622.65) | 2031.80 (102,199.61) |
| Best supportive care until death (palliative) | 84 | 3244.14 (163,180.65) | 2657.86 (133,690.51) | 2794.41 (140,558.97) | 606.42 (30,503.17) |
| Start of treatment until death, Total | 115 | 4414.08 (222,028.43) | 3191.15 (160,515.25) | 4183.44 (210,427.06) | 772.80 (38,871.87) |
The breakdown of the cost of each admission per phase of treatment was categorized (Table 2). For all patients with AML who underwent Phase 1 and Phase 2 treatments, the bulk of the expenses were from chemotherapy and other therapeutics (Table 2). This included chemotherapeutic agents, IV fluids, antibiotics and mechanical ventilator use, if applicable, among others. The admissions under phase 1 and 2 required more medications for chemotherapy and supportive medications during the duration of the management of cytopenia. The costs of blood bank and transfusion services, as well as the costs of diagnostics, were markedly lower in phase 2, compared to phase 1 treatment, as this phase required a shortened hospitalization duration and more predictable nadir and foreseeable complications.
Patients who underwent reinduction chemotherapy for relapsed disease had higher treatment costs for chemotherapy and other therapeutics (Tables 2 and 3), as the agents used were more expensive and more cytotoxic, requiring more supportive care for the duration and management of cytopenia. Albeit necessary to determine the exact direct cost of these newer agents, this figure is unfortunately an underestimate, as not all the agents being used for relapsed cases were available locally at the institution, nor can be specially purchased, as these agents have not yet been included in the Philippine National Drug Formulary.
Patients who underwent condensed HIDAC (Days 1,2 and 3) and were discharged after chemotherapy administration (average of 4 - 5 days) were readmitted on the nadir of their counts for blood transfusion support. The average cost for blood bank and transfusion services of these admissions were $150.75 (Php 7658.42). The average duration of hospital days were 9.9 days in 106 admissions for blood transfusion after consolidation chemotherapy. Since inpatient admissions for blood transfusions are not covered by Philhealth and the average total cost of admissions for these patients is $568.35 (Php 28, 872.36), this amount can be saved by an outpatient blood transfusion service, in which the patients can schedule their transfusion requirement and, at the same time, benefit from the coverage of the Philhealth reimbursement for outpatient blood transfusion services.
Unfortunately, since the option for HSCT is not available at our institution, the cost of this important treatment plan for relapsed and refractory cases was not available and was not included in the scope of the study.
These data suggest that, while it is an important cost driver in the treatment of AML, chemotherapy was able to prolong the life of an AML patient by a significant 8 months. However, the cost for both the patient and the institution for therapies for those eligible for that care is burdensome. To be able to sustain chemotherapy, patients would benefit from a health insurance that would allow beneficial coverage for the high cost of treatment. The current case rate for chemotherapy of a particular patient might be generous in terms of allowed deductible days from the allowed period, but an increase in the amount of reimbursable allowance benefit would greatly help patients, as the average expenditure for a Filipino for health alone is only 1.96% of the total cost for an average AML patient who will undergo chemotherapy.5
A limitation of this study is that, while there is a need to account for the Philhealth payments, as it is a considerable contributor to the hospital funding, the exact amount of Philhealth case rates that are directly paid to PGH was not accounted for in the duration of the study period. There were several factors as to why the total contribution of Philhealth based on the case rates was not deducted from the total cost of the AML treatment. First, the actual cost cannot be accounted for, as the auto-credit payment does not include the No Balance Billing (NBB) scheme. Similarly, while the actual date, time, amount and duration of payables from Philhealth for an individual's reimbursement to the hospital cannot be accounted for based on the bill's statements collected, neither can it be established whether these payables were credited to the hospital for the duration of the study. Primarily, the objective of this study was to determine the economic burden of the treatment to AML patients, from the hospital's point of view. The retrospective nature of the study precludes adequate evaluation of how much can be saved from a specific treatment to further improve future practice. In a similar manner, as the study was performed in a resource-limited setting and ideal treatment and diagnostics were not available to all patients, these contributed to the underestimation of costs.
In conclusion, patients with AML, whether newly diagnosed or having relapsed disease, represent a significant economic burden to the patients themselves and to the institution. The cost of treatment is high and increases as the disease is not controlled, with poor outcomes. When survival is considered, it seems that chemotherapy still prolongs life by several months. The available treatment options for patients with a higher prognostic risk stratification is not available at the institution (i.e., bone marrow and hematopoietic stem cell transplant), hence, this may underestimate the real-world economic impact of the burden of the disease. The total lifetime costs and outcomes for AML should be explored in future research, as current data is limited. In addition, the benefits of targeted therapies and novel agents for the treatment of relapsed and refractory disease need to be studied to assist providers in the utility of these viable treatment options after initial treatment failure. The provision for packages which will cover treatment benefits for both service and private/pay patients has the hope of alleviating the economic burden of the AML treatment to the institution and patients.
Further studies, such as prospective direct cost determination and studies which includes cost of treatment of HSCT, should be pursued. Data from these studies can predict how much can be saved by prolonging disease-free survival with upfront HSCT for relapsed and refractory cases. This could help appropriate resource allocation to provide the opportunity for ideal treatment to intermediate and high-risk AML patients.
We would like to acknowledge Dr. Michael C. Mo for his invaluable time in the analysis of the statistics and figures of this paper and for spending extra time in helping achieve a clearer structure. We would also like to express our sincerest gratitude to Dr. Erika Belinda T. Chen for her generous assistance in the data collection, and Dr. Teresita E. Dumagay for her support and encouragement to pursue this topic. My deepest appreciation to Ms. Elenita Del Rosario of the UP-PGH billing department for her generous assistance in providing information from their database.
| SUMMARY OUTPUT | ||||
| Regression Statistics | ||||
| Multiple R | 0.883852259 | |||
| R Square | 0.781194815 | |||
| Adjusted R Square | 0.768809616 | |||
| Standard Error | 101,573.393 | |||
| Observations | 113 | |||
| ANOVA | ||||
| df | SS | MS | F | |
| Regression | 6 | 3.90452E+12 | 6.50753E+11 | 63.07486908 |
| Residual | 106 | 1.09362E+12 | 10,317,154,156 | |
| Total | 112 | 4.99814E+12 | ||
| Coefficients | Standard Error | t Stat | p-value | |
| Intercept | 26,082.62499 | 34,651.84168 | 0.752705303 | 0.453295403 |
| Age | 361.7982249 | 665.5899038 | 0.54357529 | 0.587875177 |
| Yes Male | −35,666.3946 | 19,546.39084 | −1.824704872 | 0.070862036 |
| Hospital Days | 3171.799297 | 226.1818554 | 14.0232261 | 6.77693E-26 |
| Survival | −2183.3179 | 2200.074364 | −0.992383683 | 0.323270358 |
Each Filipino patient who is a member of the Philippine Health Insurance Corporation (PhilHealth) can avail himself or herself of a reimbursement benefit based on the case rate. Newly diagnosed AML patients, can avail themselves of $273.62 (Php 13,900) as the first medical case rate, and $143.30 (Php 7280) as the procedure case rate, which if claimed as 1st rate and 2nd rate, respectively, will both cover 100% deduction and reimbursement per admission. The remaining amount shall be charged as out of pocket to the beneficiary, except in cases where the NBB policy applies. A patient with AML, will only be able to avail himself or herself of the medical case rate once because of the single period of confinement rule.
The case rate amount for chemotherapy is equivalent to one cycle of chemotherapy. One cycle of chemotherapy is equivalent to 2 days deduction from the 45-day benefit allowance. Under these rules and regulations, each patient will have an available 22 periods of confinement, where they can deduct the case rate amount to the total number of 45 days to avail themselves of the allowable benefit. Each cycle of chemotherapy is equivalent to two (2) days of deduction from the 45-day allowable benefit per year, regardless of the number of days of confinement per cycle. Considering one chemotherapy session (based on the procedure case rate) will provide $143.30 (Php 7280) per 2-day period per cycle deduction and reimbursement, and the patient will avail himself or herself of, and consume the 45-day period allowance, a patient's Philhealth membership will allow him or her to benefit from a total of $ 3224.40 (Php 163,800) for the entire course of the chemotherapy treatment. An additional one-time $273.62 (Php 13,900) deduction allowance for newly diagnosed cases can be reimbursed based on the single period of confinement (SPC).
Based on the data derived from this study, the Philhealth allowance for each chemotherapy session allows only 5.81% coverage of the mean cost of an average remission induction chemotherapy, which costs US$ 2489.84 (Php 125,239.29). For private patients, who pay an additional average of US$ 1197.50 (Php 60,234. 42) for room and board, this case rate for chemotherapy provides an allowance benefit of only 3.92%. Suppose a patient gets admitted 22 times and consumes all the allowance benefit for the entire course of his/her treatment, it will only cover 48%, or $ 3224.40 (Php 163,800. 00) of the total cost and Php 338,500.40 for a patient who underwent standard of care. The remaining expenses will be charged to the institution, if the patient is admitted as a service case, in which the NBB is being applied. However, for private patients, this will cover 41% of the total cost per patient for all admissions under standard of care, provided all admissions are tagged with a case rate as chemotherapy. (Certain admissions were for blood transfusion on the nadir of cytopenia post-chemotherapy. These admissions cannot benefit from the reimbursement, as no chemotherapy was provided during the admission).