Convalescent Plasma therapy is one of the therapeutic strategies that has been used for patients with the Covid-19 disease. Implementing a program with national extension to supply hospitals with this blood component is a great challenge mainly in a middle-income economy.
ObjectivesOur objective was to develop and implement a Covid-19 Convalescent Plasma Program which met established quality standards and was adapted to a reality of limited resources.
MethodsA multicentric convalescent plasma collection program was developed and implemented, based on four main sequential procedures: selective donor recruitment, pre-donation antibody screening (Anti-SARS-CoV-2- Chemiluminescence IgG Abbott), convalescent plasma collection by apheresis or whole-blood processing and distribution to the hospitals according to local demand.
ResultsFrom the 572 candidates submitted to the pre-donation antibody screening, only 270 (47%) were considered eligible for plasma donation according to the established criteria. Higher levels of total antibody were associated with the donor age being above 45 years old (p = 0.002), hospital admission (p = 0.018), and a shorter interval between the diagnosis of the SARS-CoV-2 infection and plasma donation (p < 0.001). There was no association between the ABO and Rh blood groups and their antibody levels. Of the 468 donations made, 61% were from the collection of whole-blood and 39%, from apheresis. The Covid-19 Convalescent Plasma units obtained were distributed to 21 different cities throughout the country by air or ground transportation.
ConclusionThe implementation of a Covid-19 Convalescent Plasma program in a continental country with relatively scarce resources is feasible with alternative strategies to promote lower cost procedures, while complying with local regulations and meeting quality standards.
In late 2019, patients presenting an atypical pneumonia gained attention in Wuhan, China. A few days later, the SARS-CoV-2, a novel coronavirus, was identified as a viral etiology of the pulmonary disease, now called COVID-19, and its infectivity and spread capacity rapidly produced a global emergency. The World Health Organization (WHO) declared a pandemic on March 11, 2020. By March 2021, one year later, the virus was detected in almost every country around the globe, with more than 120 million people infected and almost 2.5 million deaths.1
In this devastating scenario, with insufficient understanding of the disease and no evidence-based treatment available, several approaches have been considered, including the transfusion of plasma obtained from patients who had recovered from the SARS-CoV-2 infection. The Convalescent Plasma transfusion, a form of passive immunity, is not a novel therapy and has been used for the treatment of other virus infections, such as Influenza, Ebola, MERS and SARS, with conflicting data about its efficacy. Early reports from China showed favorable outcomes following the administration of convalescent plasma to patients with severe and life-threatening COVID-19.2–4 Many centers worldwide have promoted clinical trials to evaluate the safety and efficacy of the Covid-19 Convalescent Plasma (CCP) against SARS-CoV-2. Simultaneously, complying with local regulations, programs have been established in many countries to promote the distribution and use of the COVID-19 convalescent plasma as an expanded access therapy.
In this paper, we will describe our challenge in developing and implementing a nation-wide CCP program in a middle-income country with a continental geographic area and a poor regular blood donation culture among its population, as well as its impact on logistics due to the pandemic.
MethodsIn a multicenter, prospective and observational study, all donors submitted to CCP collection from the 14 blood collection facilities distributed among 12 Brazilian cities were analyzed. All donations were altruistic and voluntary. The donor samples were sent from the collection sites to a central laboratory for characterization and analysis. The plasma units collected by apheresis and obtained by the processing of whole blood were collected at each site, processed, stored in a regional center and distributed to the hospitals by ground or air transportation according to local demand. Medical decisions about treating the patient with COVID-19 in the 205 hospitals did not suffer any interference by the program. The collected data were stored in the institution's secure database. All data were analyzed anonymously.
Donor recruitment and eligibilityThe recruitment of candidates for convalescent plasma donations involved multiple approaches. They were recruited from the hospitals and clinical pathology laboratories where they had been diagnosed with Covid-19 and from the general population. The recruitment tools applied were e-mail, mobile phone messages, websites, social media, television and radio. The patients who had recovered from COVID-19 were encouraged to contact one of the collection units to go through a pre-donation screening. The CCP donors were to meet all national requirements established for the convalescent plasma and regular blood donation.5,6 Men and non-previously pregnant women with confirmed prior SARS-CoV-2 infection were considered eligible for the convalescent plasma donation 30 days after the complete resolution of the symptoms from Covid-19. Donors with previous intensive therapy care and severe pulmonary impairment were not accepted for plasma/blood donation. To increase the likelihood of having appropriate antibody levels in the donated plasma, according to previous studies of the antibody kinetics of the SARS virus, donations were allowed only until three months after the date of the documented infection.7 All donors gave informed consent for their participation in the program and donated plasma or whole blood only once.
Inclusion criteria- -
Age: > 18 and < 60 years old
- -
Men or non-previously pregnant women
- -
30 days after the complete symptoms resolution from mild or moderate SARS-CoV-2 infection or 30 days after the diagnosis of the previous asymptomatic infection, until three months after the diagnosis confirmation date.
- -
Laboratorial evidence of previous SARS-Cov-V-2 infections (IgM, IgG or RT-PCR)
- -
Meeting of all requirements established for regular blood donations
To be classified as a potential donor and submitted to the screening test, eligible candidates were required to show a laboratory result that proved previous SARS-CoV-2 infection (IgM, IgG or RT-PCR). Before each plasma or blood donation, the classified candidates were submitted to an Anti-SARS-CoV-2- Chemiluminescence IgG Abbott (Chicago, US) antibody screen. Tests were performed in accordance with the manufacturer's instructions. Previously published data showed that an optical density (OD) above 3.5 is associated with neutralizing antibody titers ≥ than 1:80.8,9 Thus, we interpreted our results as negative when the OD was < 1.4, as a weak positive with the OD between 1.4 and 3.5 and as a strong positive with the OD > 3.5). Only candidates with strong Anti-SARS-CoV-2- IgG Abbott positive results were invited for CCP donation. As donors were not submitted to a new RT-PCR at admission, collection sites prevented close contact between the plasma donors and regular blood donors as much as possible according to local conditions and particularities.
Plasma collection and productionConvalescent Plasma units were collected by apheresis or obtained by the processing of whole blood. The apheresis collection was performed with the Trima Accel (Terumo BCT, USA) or Amicus (Fresenius Kabi, USA) equipment. The anticoagulation was obtained with Anticoagulant Citrate Dextrose Solution, Solution A (ACD-A). The maximum volume collected per procedure was 600ml, according to the donor body weight. The units collected were split into 2 or 3 units of 200 ml each. The whole-blood collections were performed for the production of Red Blood Cells (RBCs) and Convalescent Plasma. The volume of plasma units derived from whole blood varied from 200 to 250 ml. The RBCs were incorporated into the regular blood bank inventory and used for transfusion as needed. The CCP freezing was performed until 6 hours from the collection and stored at a temperature below 20°C negative.
The choice between the apheresis or whole-blood donations was based on the CCP demand, the donor venous access, the history of previous donations (first time or repeat donor) and the availability of the disposable apheresis and technology.
Statistical analysisCategorical variables were summarized with frequencies and percentages, while continuous variables were summarized with the mean ± standard deviation or median. The variables were then assessed for the IgG anti-SARS-CoV-2 level, based on the Optical Density obtained with the Abbott ELISA testing using the Student's t-distribution,
Two-sided p-values < 0.05 were considered statistically significant.
All analyses were conducted in the NCSS 2020 software (www.ncss.com).
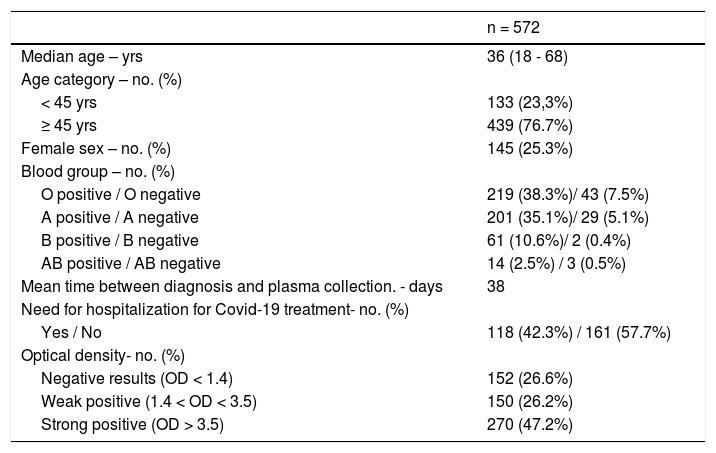
ResultsPotential CCP donors had a mean age of 36 years old, were predominantly males (74.7%) and the majority (57.7%) had mild or moderate disease, not demanding hospitalization. The mean interval between the diagnosis of the SARS-CoV-2 infection and the CCP donation was 38 days. The ABO and Rh blood group distribution in the CCP potential donors was similar to that found in the general population. (Table 1).
Characteristics of CCP donors.
OD: optical density; yrs: years.
Almost half of the 572 eligible CCP candidates were considered strong positives (270/572, 47.2%), 26.2% (150/572) were weak positives and 26.6% were considered negative (152/270). (Table 1).
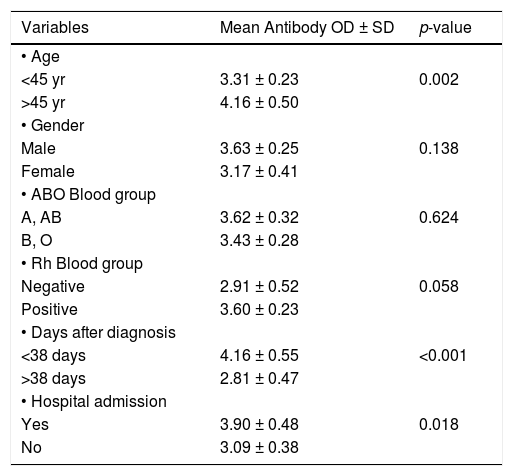
Table 2 shows the antibody level according to the epidemiological and clinical donor characteristics. Age above 45 years old, need for hospitalization and an interval lower than 38 days from the SARS-CoV-2 diagnosis to the blood donation were significantly associated with higher antibodies levels. The donor gender and the ABO and Rh blood groups were not associated with differences in antibody levels.
. Anti-SARS-CoV-2 results from CCP donors according to clinical characteristics.
OD: optical density; yrs: years; SD: Standard Deviation
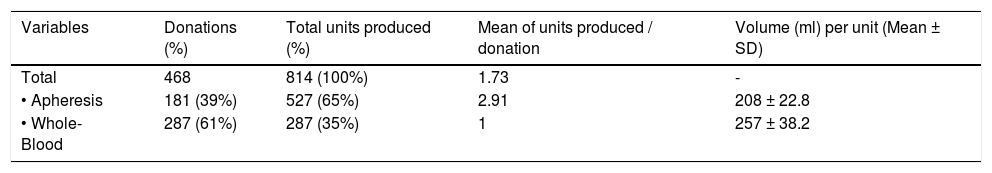
The characteristics of the 814 CCP units collected are reported in Table 3. The whole blood collection was responsible for most of the donations (287/468), while apheresis was responsible for the majority of the CCP units obtained (527/814). The mean number of units collected per apheresis procedure was 2.91 and the median volume of CCP units, 208 and 257, for the apheresis and whole blood plasma units, respectively.
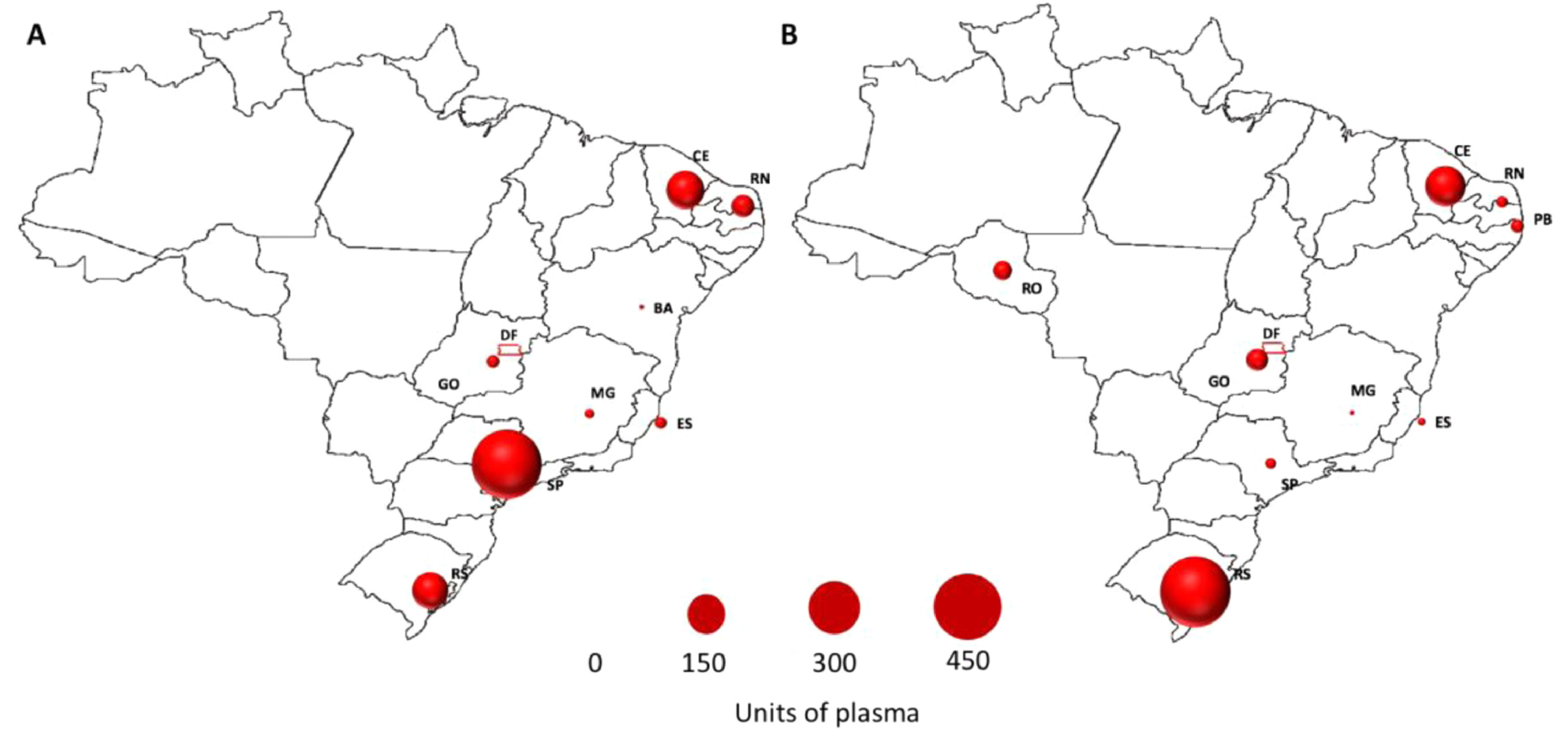
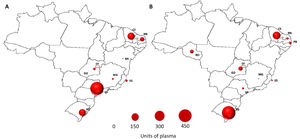
Convalescent plasma distributionThe plasma collection was performed at 14 collection sites, but there were significant differences among them, regarding the blood donor recruitment capacity and units of plasma collected. The States of São Paulo (57%), Ceará (17%) and Rio Grande do Sul (15%) collected over 75% of the total plasma units (Figure 1 A). The CCP units were stored in the blood banks located in central regions and distributed to the hospitals as soon as transfusion requests were received.
The hospital that desired access to a CCP unit were required to sign a formal agreement for its use, based on controlled studies or compassionate therapy. Each institution developed and provided its own protocols of clinical indications for the convalescent plasma therapy.
The States of Rio Grande do Sul (62%) and Ceará (20%) were responsible for more than 80% of the plasma supplied, serving 21 municipalities across the country (Figure 1 B). The collection and transfusion of the CPP often did not take place in the same region. Therefore, in order to provide sufficient CPPs for those institutions and locations, regular shipments and transportation from one center to another were arranged daily.
DiscussionConvalescent plasma has a long history of utilization in patients suffering from a variety of infectious diseases. The current Covid-19 pandemic represents a unique opportunity to put convalescent plasma to the test as a prophylactic intervention and a therapeutic treatment for established disease.2,10,11
The blood donor recruitment has been a hurdle in low- and middle-income countries. While high-income countries (HIC) have almost 4 to 5% of the population donating blood regularly, the middle-income country Brazil struggles to reach 2% of the population as regular blood donors and depends on voluntary non-remunerated donors to maintain its blood supply.12 As the eligibility criteria for CCP donations are even more restrictive, the recruitment of plasma donors creates a new and greater challenge. For the recruitment of potential plasma donors, we applied many different communication tools. Nevertheless, it was difficult to reach the required and adequate number of donors.
As its effectiveness has not yet been proved, the convalescent plasma is still not included in the routine of the blood components production process, nor is it standardized. Regulatory agencies have provided guidance based on scarce evidence, but their specifications are still a matter of debate.13 Currently, there are reports showing contradictory results regarding the efficiency of CCP, probably limiting its benefit to its early use, with high antibody titers in high-risk patients.14–16
In most countries, a time deferral policy, ranging from 14 to 30 days after the symptoms resolution, has been used for the eligibility for plasma donation. According to Brazilian regulations, it is possible to donate the CCP after 14 days of the complete resolution of the symptoms, but complying with special arrangements at collection sites to avoid the contact of plasma donors with regular blood donors. After 30 days, the candidate can either donate whole blood or CCP with no further restrictions. In our program, to increase the probability of potential donors not being infectious, once we did not perform a new RT-PCR, and to allow enough time for the antibody response, the choice was for an interval of 30 days between the complete resolution of symptoms and the plasma donation. In addition, the collection sites avoided close contact between the donors with previous known SARS-CoV-2 infections and the regular whole blood donors.
Apheresis has been the main technology used for convalescent plasma collection around the world. It has some advantages, such as making it possible to collect larger volumes of plasma per donation, providing 2 to 3 units of CCP from each donor, as well as reducing the intervals between two collections. However, the higher cost, the necessity for a trained staff and an adequate venous access and the viability of disposables and equipment are limiting factors.2 On the other hand, whole blood collection and the processing of the convalescent plasma are readily available and less expensive, not to mention the possibility of also producing one RBC. However, this process demands a longer interval for the next donation and each donation provides only a small amount of convalescent plasma. Given our reality, our program included the production of convalescent plasma from whole blood donations and apheresis, each one representing 61% and 39% of all donations, respectively.10 The minimal interval of 30 days after the complete recovery and the production of CCP from whole blood were two choices in our program that were necessary for our reality and that enabled its application and continuity.
The issues surrounding Anti-SARS-CoV-2 antibodies are another important ongoing discussion. There is a consensus that the effectiveness of the CCP depends on the presence of specific antibodies. Antibodies against the Spike protein or the Nucleocapsid of the virus and especially neutralizing antibodies have been assumed to be desirable.17 Neutralization assays are limited in availability and throughput, requiring biosafety level 3 facilities and skilled labor. Since such assays are often unavailable, one alternative is to perform the test later on a stored sample, or to perform another test to detect the presence of the anti-SARS-CoV-2 antibody prior to issuing the plasma unit for transfusion. There are many options based on immunoassays (Elisa or Chemiluminescence), with lower costs, faster results and greater availability. Studies have been published demonstrating a correlation between immunoassay tests with neutralization activity.2,8 Many centers have been using the OD results obtained by immunoassays as a surrogate marker for the neutralization activity.18,19
Mendrone-Junior demonstrated a good correlation between immunoassays and nABs titers and concluded that immunoassays can be used as a predictor of the high neutralizing antibody (nAB) titers with 76.1% of accuracy of nABs ≥ 1:160 with S/CO ≥ 4.57, using the Chemiluminescence IgG Abbott test.19
Jääskeläinen, also demonstrated the correlation between the OD of the Anti-SARS-Cov-2 IgG assay (Abbott) with the microneutralizing activity.8,9 In this study, the immunoassay assay demonstrated a correlation between an nAB titer above 1:80 when the S/CO was higher than 3.5.
Although the clinical importance of this data is still unknown, due to the restricted access to the neutralization assay we only considered potential donors with an OD above 3.5 eligible for plasma donation.8,9 Although higher values of OD are correlated with a higher positive predictive value of an nAB titer ≥ 1:160, our choice was for a cutoff point of 3.5 in OD for a better balance between levels of the nAB and the lower loss of potential donors, reenforced by the data of Mendrone-Junior et al., showing that approximately 20% of the potential donors with a positive IgG test, but with a S/CO lower than 4,57, have an nAB ≥ 1:80.
In our program, all candidates were required to have a documented infection (IgM, IgG or RT-PCR) to be considered eligible to perform antibody screening. Interestingly, even in this previously selected population, 26.6% of the screened individuals were negative for IgG (OD < 1.4), 26.2% showed a weak positive (OD ≥ 1.4 and < 3.5) and only 47.2% of the tests performed provided eligible CCP plasma donors (strong positive OD ≥ 3.5). Some factors can be associated with these data, either alone or in combination. The anti-SARS-Cov-2 IgG Abbott assay for spike proteins has a reported specificity of 95.1% and sensitivity of 80.5% to identify the IgG.8,9 Recent reports have shown that antibody production may be correlated with disease severity, severe disease being associated with higher antibody titers, while less severe disease was associated with lower IgG titers or even not inducing seroconversion.20 Another critical possibility is the occurrence of false positives in the tests used for the diagnosis of the disease, particularly with the IgG and IgM rapid tests. As soon as the pandemic spread, many rapid tests were developed, with no adequate assessment of their analytical performance.21,22
It has been reported that the antibody levels in patients that had died from Covid-19 were significantly lower than those who had survived, suggesting that the antibody response may have an essential role in the patient's recovery.20 Some studies have demonstrated other variables associated with high levels of antibodies in addition to the severity of the disease, such as age and a shorter interval of the complete resolution of the symptoms.20,23,19 Our results corroborate these findings. Donors aged over 45 years old (p = 0.002), with moderate disease needing hospital admission to treat Covid-19 (p = 0.018) and screened before 38 days from diagnosis (p < 0.0001), had a significant higher level of antibodies (IgG) directed against SARS-CoV-2.
Recent reports have been associating the patient ABO blood group with disease severity. Individuals with groups A and AB have a higher disease severity than O and B.24 This phenomenon is not fully understood, but some authors speculate that the presence of a natural anti-A would have a protective effect (O and B groups).24–26 Another hypothesis is that specific antibodies directed against SARS-CoV-2 would be produced with different intensities, depending on the patient's blood group. In our study, there was no significant association between the ABO blood group and the Anti-SARS-CoV-2 IgG antibodies levels.
According to an IATA report in April 2020, there was a 95% and 65% decrease in the air transport of passengers and cargo, respectively.27 The logistical options available for transporting blood samples from the collection sites to the central laboratory and distributing blood components from the regional center to the hospitals were minimal. In addition, as the plasma therapy is still under investigation, there was no uniform adherence across the country. Some centers have used this therapy even without a collection site in their region (e.g., Porto Velho in the State of Roraima, 2,500 km. from the nearest blood collection site). Therefore, to overcome this situation of high demand in areas with insufficient collection or no collection of CCP, in the context of limited air transport, it was necessary to establish daily routes between the blood banks and the hospitals served. In these centers with insufficient or no collection, the challenge was to ensure that the demand for the CCP would not outstrip the availability of the product. This scenario has been reported in the entire world, mainly in countries where the CCP therapy became the standard of care.28 The development of clinical guidelines and an adequate patient selection would be critical in minimizing this problem.
Although the CCP appears to have limited effectiveness, the reported program using a selective donor recruitment, different collection techniques, regional blood distribution centers and transportation diversification can be used as a model for health institutions responding to other public emergencies.
ConclusionIn conclusion, herein we evaluated the feasibility and implementation of a CCP program in an upper-middle income economy with a limited-resource setting, meeting the established international and local standards, using alternatives, such as a pre-donation antibody IgG screening, multicentric whole-blood donations and a nationwide CCP transportation and distribution. Our results also validated previously reported data related to the association of higher antibody levels and disease severity and demonstrated the importance of age and time from diagnosis until the antibody screening.28 Further clinical trials should be performed to evaluate the CCP safety and efficacy.
We acknowledge the altruistic contribution from blood donors all over Brazil. We would also like to thank all teams involved in the donor recruitment, blood collection, laboratory analysis and component distribution logistics. This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
Duarte GC designed the research study, performed the research and wrote the paper. Simoni V, Haddad R, Toledo RSM and Ottoboni MAP performed the research. Ribeiro GN and Moschen M analyzed the data and wrote the paper. Mendrone-Junior A and Langhi DM wrote the paper. All authors agreed upon the final manuscript.