von Willebrand’s disease (VWD) is the most common inherited bleeding disorder. The 1-desamino-8-d-arginine vasopressin (DDAVP) is the treatment of choice for most responsive patients with VWD. The aim of this study was to evaluate DDAVP use in the management of VWD.
MethodWe implemented a survey targeting medical doctors involved in the management of VWD in Brazil. Data was collected during a national congress on Hematology in November 2017.
Main resultsA total of 51/80 (63.8%) questionnaires were collected. Most participants (76.2%) were hematologists who assisted adult patients and approximately 60% worked at hemophilia treatment centers (HTCs). Approximately half of participants who reported treating patients with VWD, assisted on average, less than 5 patients per month, and approximately 60% declared not having used any DDAVP for treating VWD in the previous year. However, most participants (70%) prescribed FVIII-containing VWF concentrate (VWF/FVIII) for 1–10 patients in the previous year. More than 80% of the participants recognized the main indications for DDAVP. Physicians who recognized indication for DDAVP for type 1 VWD more often had prescribed DDAVP in previous year (p = 0.03). Barriers for prescribing DDAVP varied and included unavailability of laboratory facilities and consumables for DDAVP testing and lack of skills on its prescription.
ConclusionThe DDAVP is currently underused in Brazil, as opposed to the excessive use of VWF/FVIII in VWD patients. We suggest the adoption of measures targeting educational and auditing programs. Furthermore, availability of laboratory reagents is needed to evaluate response and increment the correct use of DDAVP.
The von Willebrand’s disease (VWD) is the most common inherited bleeding disorder (IBD), with an estimated prevalence of approximately 1% in the general population.1,2 The prevalence of symptomatic VWD has been estimated to be 1:1000 to1:10,000 individuals.3,4 The diagnostic criteria of VWD include a personal bleeding history associated with laboratory abnormalities and family history of bleeding.5 The VWD classification consists of types 1, 2 and 3, of which type 2 has 4 subtypes, 2A, 2B, 2 M and 2N.4,6 Types 1 and 3 are quantitative defects of the von Willebrand factor (VWF) and type 2 and its subtypes are functional defects.4,6 The diagnosis of VWD is challenging, due its heterogeneity, variations in diagnostic criteria and requirement for complex laboratory tests.4–6
The standard treatment of VWD consists of increasing VWF levels above 50% in the course of bleeding episodes or for prophylaxis in case of surgery and other interventions.7–10 Most bleeding episodes in patients with VWD can be effectively treated with desmopressin acetate [DDAVP (1-desamino-8-d-arginine vasopressin)], a synthetic analog of the natriuretic hormone.11 The DDAVP induces the release of the von Willebrand factor (VWF) from its storage sites in the endothelial cells and increases plasma levels of factor VIII (FVIII).11–13 In patients with VWD, DDAVP is the treatment of choice for mild to moderate bleeding and for prophylaxis of minor surgeries in responsive patients, except for subtype 2B and type 3,5–10,14 A good response to DDAVP has usually been defined as an increase in plasma VWF and FVIII levels of three to five times above the basal levels within 30−60 min after intravenous injection, or 60−90 min after subcutaneous (SC) injection of DDAVP, at a dose of 0.3 µg/kg.7–11 The type and severity of VWD, documented response9 and potential risks of therapy need to be considered. For non-responding patients, for those presenting VWD types 2B and 3, or any subtype with severe bleeding or requiring prophylaxis for major surgeries, factor VIII-containing VWF (VWF/FVIII) concentrate is recommended.7–10
In Brazil, health care for people with hemophilia, VWD and other IBDs is publicly funded and patients are attended at hemophilia treatment centers (HTCs) by a multidisciplinary team.15 According to the Brazilian Ministry of Health data, in 2016, a total of 2,808/7811 patients 35.9%) who are registered with VWD used one or more types of procoagulant agents for treatment of any bleeding episode.16 Most of these patients (52.4%) used VWF/FVIII concentrates, followed by 40.3% and 7.3% who used tranexamic acid and DDAVP, respectively.16 This finding has alerted us to a possible underuse of DDAVP in Brazil and led us to seek for reasons why DDAVP has not been more frequently prescribed.
Therefore, the aim of this study was to evaluate the use of DDAVP by medical doctors in the management of VWD patients. We also aimed to investigate the reasons for not using the product when it was indicated and the awareness of participants of the indications and contraindications of DDAVP. For this, we implemented a survey targeting medical doctors working with VWD patients.
MethodsStudy design, settings, inclusion and exclusion criteriaThe present study is a survey. A questionnaire was applied to medical professionals who attended the Brazilian Congress of Hematology and Hemotherapy in Curitiba, Parana, Brazil in November 2017. The survey was printed and participants were invited to participate during the Congress. The questionnaire was applied for two consecutive days (November 9 and 10, 2017). One interviewer identified the physicians by the badge “Medical doctor” randomly in the hemostasis sessions during the congress. Participation in the survey was voluntary and anonymous. The questionnaire was returned at the end of the session.
The inclusion criteria required the participant to be an adult or pediatric hematologist or any physician who had been involved in the treatment of VWD in the previous 12 months.
VariablesThe survey comprised a standardized questionnaire containing 14 questions, some of which were single response questions, others permitted multiple responses. It was designed by the authors. The questions included information on: (i) demographic and professional data of participants and their institutions; (ii) practice in the treatment of patients with VWD; (iii) quantification of the mean of patients with von Willebrand disease treated per month; (iv) information related to DDAVP prescription, administration route (subcutaneous or intravenous) in the last 12 months; (v) use of VWF/FVIII concentrate in the last 12 months; (vi) knowledge about consensual indications and contraindications of DDAVP use in VWD, and; (vi) reasons for not using DDAVP while indicated. Lastly, there was a space for free comment and opinion on any aspect of the survey and related issues.
We defined the following conditions for the consensual indication use of DDAVP in VWD: (i) DDAVP test to check response in newly diagnosed VWD patients, except type 3 and subtype 2B, and; (ii) VWD type 1 and 2 (except subtype 2B) with good response to the DDAVP test. The consensual contraindication use of DDAVP in VWD was defined as: (i) hyponatremia, seizures or hypertension or children under 3 years; (ii) VWD type 2 with thrombocytopenia; (iii) coronary artery disease, and; (iv) renal failure. We also included pregnancy in a question as a contraindication to DDAVP use in order to check the opinion of Brazilian physicians on this controversial matter.
Statistical analysisIn the descriptive analysis of the categorical variables, the number of events and their respective percentages were calculated. Free answers were reported grouped by the similarity between them. For the comparisons between the responses from participants who were using DDAVP (Group 1) versus those not using DDAVP (Group 2), the Fisher's exact and chi-square tests were performed. Test results were considered statistically significant if the p value was less than 0.05. Statistical analyses were performed using the Statistical Package for Social Sciences software (IBM-SPSS, Chicago, USA), version 18.0.
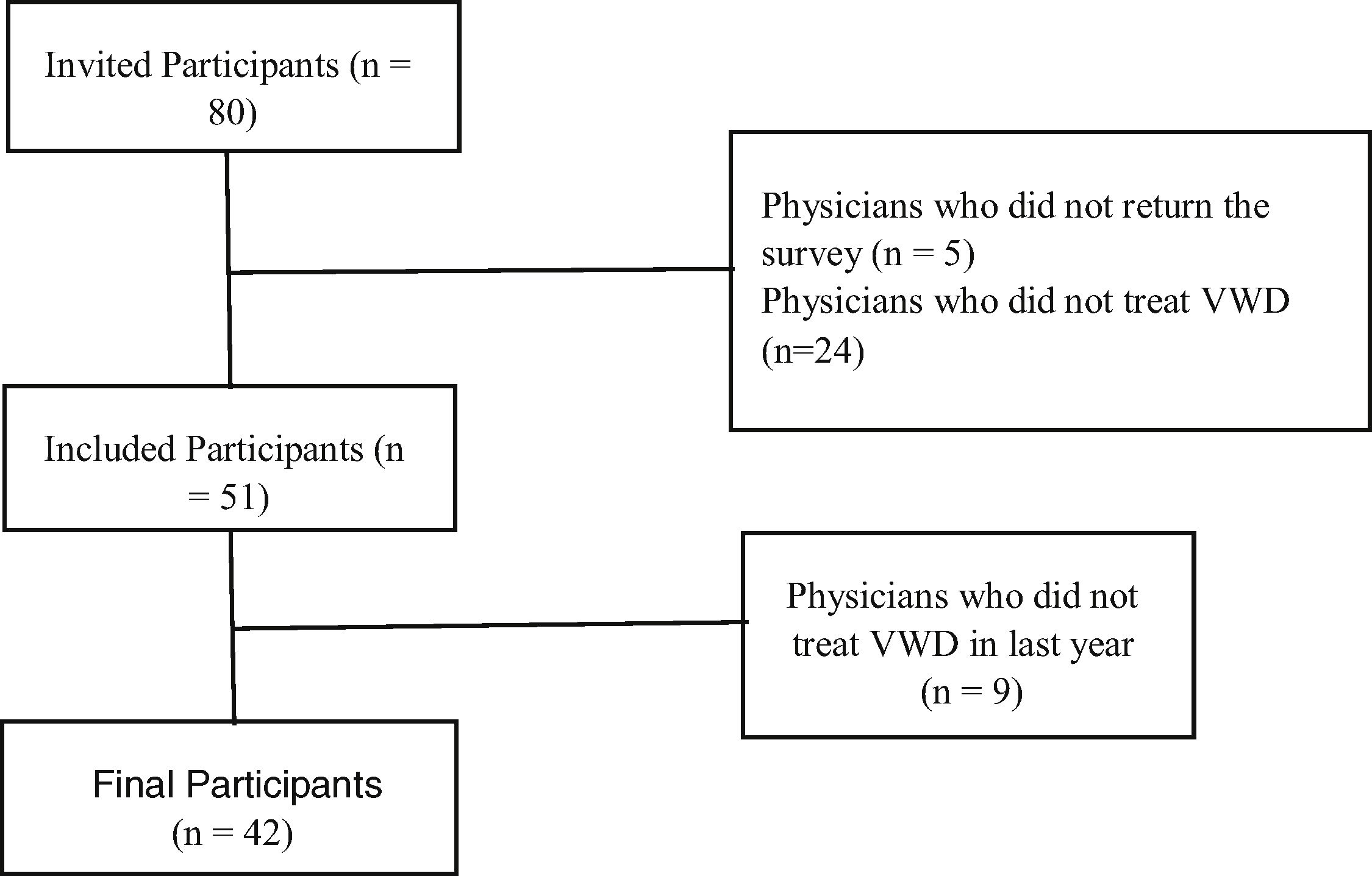
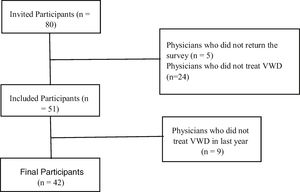
ResultsParticipantsThe survey was distributed to a total of 80 participants during the congress. Surveys were recovered from 51/80 (63.8%) participants, of whom 42 (82.4%) reported current practice in the treatment of patients with VWD (Fig. 1).
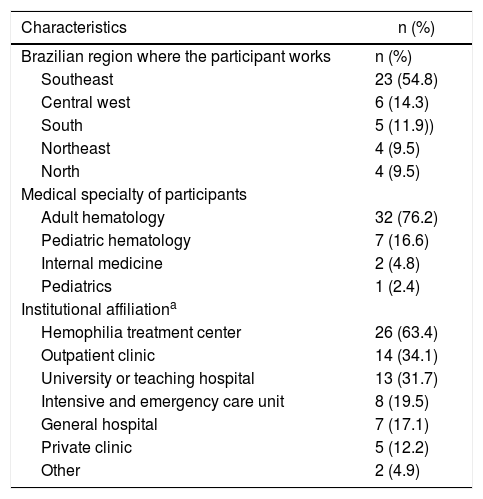
Most participants (32/42; 76.2%) who reported treating VWD patients in the last year were hematologists who assisted adult patients and 63.4% (26/41) worked at an HTC (Table 1). Most participants (54.8%) were from southeastern Brazil. The detailed characteristics of the participants are shown in Table 1.
Characteristics of the participants included in the study.
| Characteristics | n (%) |
|---|---|
| Brazilian region where the participant works | n (%) |
| Southeast | 23 (54.8) |
| Central west | 6 (14.3) |
| South | 5 (11.9)) |
| Northeast | 4 (9.5) |
| North | 4 (9.5) |
| Medical specialty of participants | |
| Adult hematology | 32 (76.2) |
| Pediatric hematology | 7 (16.6) |
| Internal medicine | 2 (4.8) |
| Pediatrics | 1 (2.4) |
| Institutional affiliationa | |
| Hemophilia treatment center | 26 (63.4) |
| Outpatient clinic | 14 (34.1) |
| University or teaching hospital | 13 (31.7) |
| Intensive and emergency care unit | 8 (19.5) |
| General hospital | 7 (17.1) |
| Private clinic | 5 (12.2) |
| Other | 2 (4.9) |
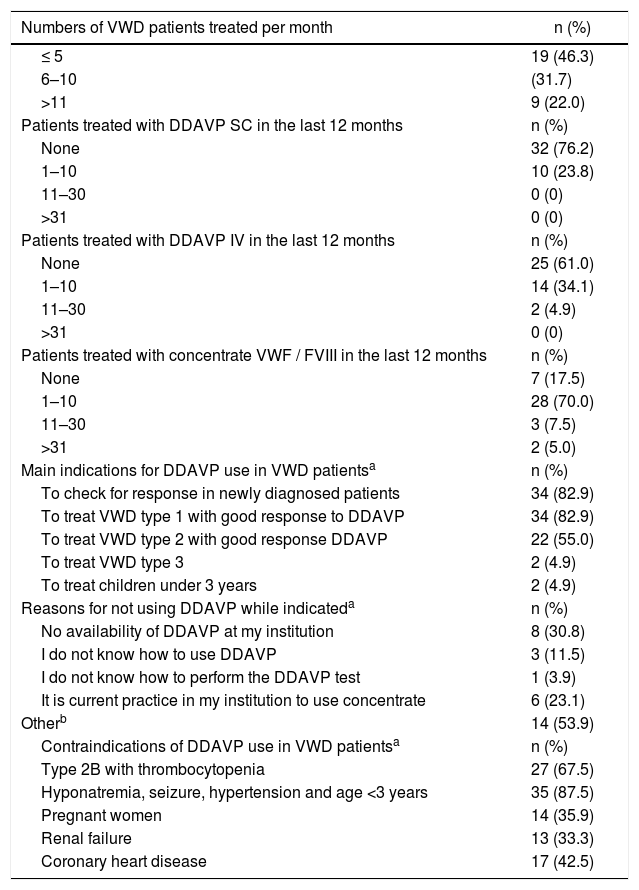
A total of 42/51 participants (82.4%) who answered the questionnaire reported involvement in the treatment of VWD in the previous year (Fig. 1). A few participants did not answer some questions (Table 2).
Characteristics of the treatment of von Willebrand’s disease.
| Numbers of VWD patients treated per month | n (%) |
|---|---|
| ≤ 5 | 19 (46.3) |
| 6–10 | (31.7) |
| >11 | 9 (22.0) |
| Patients treated with DDAVP SC in the last 12 months | n (%) |
| None | 32 (76.2) |
| 1–10 | 10 (23.8) |
| 11–30 | 0 (0) |
| >31 | 0 (0) |
| Patients treated with DDAVP IV in the last 12 months | n (%) |
| None | 25 (61.0) |
| 1–10 | 14 (34.1) |
| 11–30 | 2 (4.9) |
| >31 | 0 (0) |
| Patients treated with concentrate VWF / FVIII in the last 12 months | n (%) |
| None | 7 (17.5) |
| 1–10 | 28 (70.0) |
| 11–30 | 3 (7.5) |
| >31 | 2 (5.0) |
| Main indications for DDAVP use in VWD patientsa | n (%) |
| To check for response in newly diagnosed patients | 34 (82.9) |
| To treat VWD type 1 with good response to DDAVP | 34 (82.9) |
| To treat VWD type 2 with good response DDAVP | 22 (55.0) |
| To treat VWD type 3 | 2 (4.9) |
| To treat children under 3 years | 2 (4.9) |
| Reasons for not using DDAVP while indicateda | n (%) |
| No availability of DDAVP at my institution | 8 (30.8) |
| I do not know how to use DDAVP | 3 (11.5) |
| I do not know how to perform the DDAVP test | 1 (3.9) |
| It is current practice in my institution to use concentrate | 6 (23.1) |
| Otherb | 14 (53.9) |
| Contraindications of DDAVP use in VWD patientsa | n (%) |
| Type 2B with thrombocytopenia | 27 (67.5) |
| Hyponatremia, seizure, hypertension and age <3 years | 35 (87.5) |
| Pregnant women | 14 (35.9) |
| Renal failure | 13 (33.3) |
| Coronary heart disease | 17 (42.5) |
Of the participants who reported current management of patients with VWD, approximately half (19/41; 46.3%) assisted, in average, less than 5 patients and approximately one-quarter assisted, on average, more than 11 patients with VWD per month (Table 2).
The majority of the participants (32/42; 76.2%) did not report the use of any subcutaneous DDAVP, 15 mcg/mL, in the last 12 months for the treatment of VWD. A total of (25/41; 61.0%) reported no use of intravenous DDAVP at 4 µg/mL. On the contrary, most participants (28/40; 70.0%) had prescribed VWF/FVIII for 1–10 patients in the previous 12 months (Table 2).
Recognition of consensual indications and contra-indications of desmopressin acetateThe majority of the participants (34/41; 82.9%) agreed with the questions: “DDAVP test to check response in newly-diagnosed patients with VWD” and “VWD type 1 with good response to DDAVP test” and these are the main indications for DDAVP (Table 2). Not many participants (22/40; 55.0%), however, agreed that DDAVP is indicated for “VWD type 2 with good response to DDAVP test” (Table 2). A total of 17/40 participants (42.5%) considered these three previous items as the main indications for DDAVP.
Most participants (35/40; 87.5%) considered “hyponatremia, seizures, hypertension and children under 3 years” and (27/40; 67.5%) ‘VWD type 2 with thrombocytopenia” as the main contraindications for DDAVP use in VWD patients. ‘Coronary heart disease was considered as a contraindication by 17/40 participants (42.5%). Nearly one-third (14/39; 35.9% and 13/39; 33.3%) included “pregnancy” or “renal failure”, respectively, as contraindications for DDAVP (Table 2).
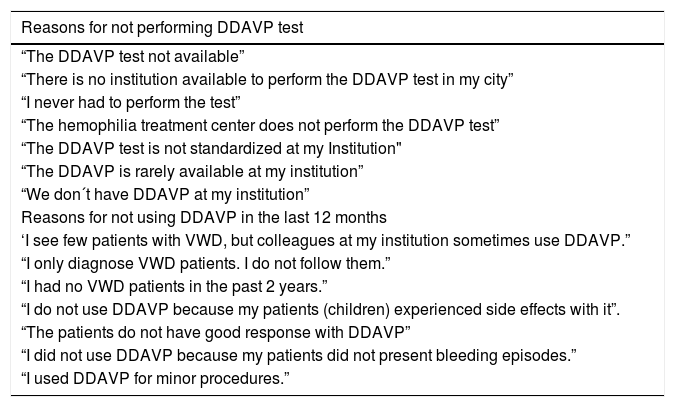
Reasons for not using DDAVP while indicatedQuestions related to the reasons for not using DDAVP while indicated involved options for four answers and additional comment. For this, we obtained 32 responses from 26 participants (Table 2), of whom one-third (8/26, 30.8%) claimed the lack of availability of DDAVP and 23.1% (6/26) justified that “It is current practice in the participant institution to use VWF/FVIII concentrate” (Table 2). Only 3/26 participants (11.5%) reported a lack of knowledge on how to use DDAVP in the clinical practice and 1/26 (3.9%) informed not being aware on how to perform the DDAVP test (Table 2). Approximately half of the participants (53.8%; 14/26) mentioned “Another reason for not using DDAVP while indicated”. As an attempt to understand these reasons, we collected qualitative answers which are shown in Table 3. The difficulties most frequently reported were related to the performance of the DDAVP test and to the low number of patients attended at the participant’s institution.
Free responses about reasons for not using DDAVP while indicated.
| Reasons for not performing DDAVP test |
|---|
| “The DDAVP test not available” |
| “There is no institution available to perform the DDAVP test in my city” |
| “I never had to perform the test” |
| “The hemophilia treatment center does not perform the DDAVP test” |
| “The DDAVP test is not standardized at my Institution" |
| “The DDAVP is rarely available at my institution” |
| “We don´t have DDAVP at my institution” |
| Reasons for not using DDAVP in the last 12 months |
| ‘I see few patients with VWD, but colleagues at my institution sometimes use DDAVP.” |
| “I only diagnose VWD patients. I do not follow them.” |
| “I had no VWD patients in the past 2 years.” |
| “I do not use DDAVP because my patients (children) experienced side effects with it”. |
| “The patients do not have good response with DDAVP” |
| “I did not use DDAVP because my patients did not present bleeding episodes.” |
| “I used DDAVP for minor procedures.” |
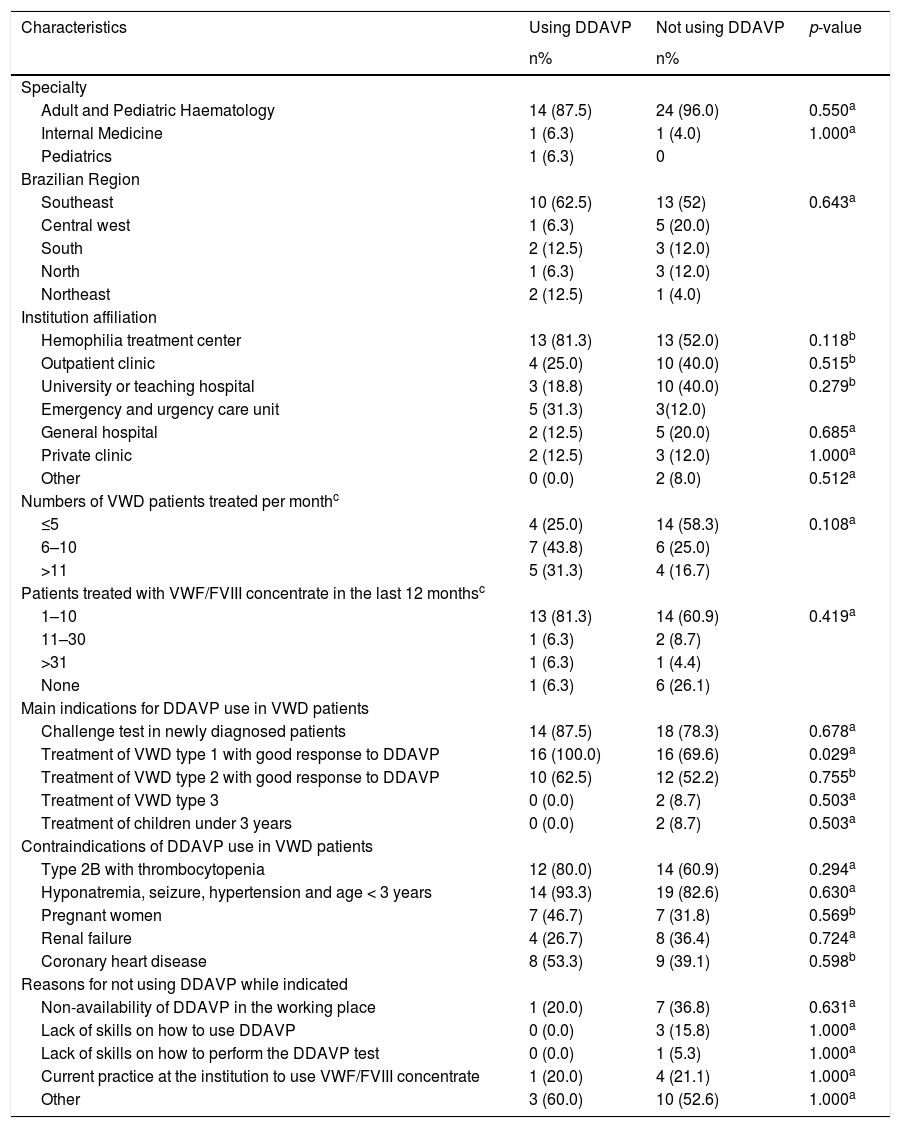
We compared the responses to this survey between participants, who reported using (Group 1) and not using (Group 2) DDAVP in the last 12 months (Table 4). A total of 16/41 (39.0%) participants belonged to Group 1 and 25/41 (61.0%) to Group 2. There was no difference between responses from the participants in Groups 1 and 2, except for the fact that the participants who recognized DDAVP as an indication for the treatment of VWD Type 1 were more often associated with the prescription of DDAVP in the last year (p = 0.03).
Comparisons between participants regarding the use of desmopressin acetate.
| Characteristics | Using DDAVP | Not using DDAVP | p-value |
|---|---|---|---|
| n% | n% | ||
| Specialty | |||
| Adult and Pediatric Haematology | 14 (87.5) | 24 (96.0) | 0.550a |
| Internal Medicine | 1 (6.3) | 1 (4.0) | 1.000a |
| Pediatrics | 1 (6.3) | 0 | |
| Brazilian Region | |||
| Southeast | 10 (62.5) | 13 (52) | 0.643a |
| Central west | 1 (6.3) | 5 (20.0) | |
| South | 2 (12.5) | 3 (12.0) | |
| North | 1 (6.3) | 3 (12.0) | |
| Northeast | 2 (12.5) | 1 (4.0) | |
| Institution affiliation | |||
| Hemophilia treatment center | 13 (81.3) | 13 (52.0) | 0.118b |
| Outpatient clinic | 4 (25.0) | 10 (40.0) | 0.515b |
| University or teaching hospital | 3 (18.8) | 10 (40.0) | 0.279b |
| Emergency and urgency care unit | 5 (31.3) | 3(12.0) | |
| General hospital | 2 (12.5) | 5 (20.0) | 0.685a |
| Private clinic | 2 (12.5) | 3 (12.0) | 1.000a |
| Other | 0 (0.0) | 2 (8.0) | 0.512a |
| Numbers of VWD patients treated per monthc | |||
| ≤5 | 4 (25.0) | 14 (58.3) | 0.108a |
| 6–10 | 7 (43.8) | 6 (25.0) | |
| >11 | 5 (31.3) | 4 (16.7) | |
| Patients treated with VWF/FVIII concentrate in the last 12 monthsc | |||
| 1–10 | 13 (81.3) | 14 (60.9) | 0.419a |
| 11–30 | 1 (6.3) | 2 (8.7) | |
| >31 | 1 (6.3) | 1 (4.4) | |
| None | 1 (6.3) | 6 (26.1) | |
| Main indications for DDAVP use in VWD patients | |||
| Challenge test in newly diagnosed patients | 14 (87.5) | 18 (78.3) | 0.678a |
| Treatment of VWD type 1 with good response to DDAVP | 16 (100.0) | 16 (69.6) | 0.029a |
| Treatment of VWD type 2 with good response to DDAVP | 10 (62.5) | 12 (52.2) | 0.755b |
| Treatment of VWD type 3 | 0 (0.0) | 2 (8.7) | 0.503a |
| Treatment of children under 3 years | 0 (0.0) | 2 (8.7) | 0.503a |
| Contraindications of DDAVP use in VWD patients | |||
| Type 2B with thrombocytopenia | 12 (80.0) | 14 (60.9) | 0.294a |
| Hyponatremia, seizure, hypertension and age < 3 years | 14 (93.3) | 19 (82.6) | 0.630a |
| Pregnant women | 7 (46.7) | 7 (31.8) | 0.569b |
| Renal failure | 4 (26.7) | 8 (36.4) | 0.724a |
| Coronary heart disease | 8 (53.3) | 9 (39.1) | 0.598b |
| Reasons for not using DDAVP while indicated | |||
| Non-availability of DDAVP in the working place | 1 (20.0) | 7 (36.8) | 0.631a |
| Lack of skills on how to use DDAVP | 0 (0.0) | 3 (15.8) | 1.000a |
| Lack of skills on how to perform the DDAVP test | 0 (0.0) | 1 (5.3) | 1.000a |
| Current practice at the institution to use VWF/FVIII concentrate | 1 (20.0) | 4 (21.1) | 1.000a |
| Other | 3 (60.0) | 10 (52.6) | 1.000a |
Bold value denotes statistically significant difference.
Table 3 shows the responses related to the non-availability of tests to check for responsiveness to DDAVP, lack of skills in the use of DDAVP and unawareness of the availability of DDAVP at the institution.
In the supplementary Table S1, free responses reinforced unavailability of laboratory facilities and consumables for testing VWF levels for the DDAVP challenge test and highlighted the problem of centralization of the VWD treatment in the comprehensive HTC as a reason for not currently being involved in the treatment of VWD.
DiscussionWe developed a survey on the use of DDAVP by Brazilian medical doctors who are involved in the management of VWD. We found that DDAVP is underused in Brazil for the management of bleeding episodes of VWD, with most participants reporting not having used DDAVP, either subcutaneously or intravenously, in the past 12 months of the survey. Instead, approximately 70% of participants reported having used VWF/FVIII concentrate to treat VWD in the past 12 months.
Most participants were hematologists who had treated patients with VWD in the previous year. More than half of them work at HTCs, mostly located in southeastern Brazil. However, only approximately 25% reported attending to a larger number of patients (>11 patients) per month.
The main reasons supplied for not using DDAVP were (i) the unavailability of DDAVP; (ii) the lack of laboratory tests to monitor the response, and; (iii) the current practice in the participant’s institution to use VWF/FVIII concentrate to treat patients with VWD. It was surprising that one of the main reasons for not using DDAVP was the unavailability of the drug. Indeed, this reflects a lack of information on the part of the participants, as DDAVP for intravenous use (4 µg/ml) has been purchased since 2004 for the treatment of patients with IBD in Brazil. More recently, the Ministry of Health (MoH) initiated the purchase of SC DDAVP (15 µg/ml). This corroborates that the underuse of DDAVP in Brazil is not related to the mode of administration of the drug. Furthermore, it is somewhat surprising that reference centers for the treatment of IBD have adopted the current practice of using VWF/FVIII concentrate to treat patients with VWD in clinical situations in which this is not the main indication. We found that most participants were knowledgeable on the main indications for DDAVP.
According to the last registry of patients with IBD in Brazil,16 there were 7811 patients with VWD, accounting for 32.2% of all IBD diagnosed in 2016. In spite of major efforts towards incrementing the appropriate diagnosis of VWD in Brazil, only 21.8% 1,703/7811 of patients have been classified according to types/subtypes. This might be partially explained by the difficulty in accessing laboratory reagents to perform tests for the VWD diagnosis, especially in some less developed Brazilian states. However, this can also be due to the lack of informed data by the HTCs in the Hemovidaweb Coagulopatias, which is the database system for patients with IBD in Brazil. In this survey, approximately one-quarter of the participants who answered the question regarding reasons for not using DDAVP while required informed the lack of laboratory reagents to monitor the DDAVP test. Therefore, this is likely to be the common reason for both problems, of the diagnosis of the disease and the performance of the DDAVP test. These limitations in the diagnosis of VWD due to the unavailability of diagnostic facilities were also reported in other developing countries, such as Pakistan and India.17–19,23
Currently, for the treatment of VWD in Brazil, there are available tranexamic acid, DDAVP for intravenous (4 µg/ml) or subcutaneous (15 µg/ml) use and plasma-derived VWF/FVIII concentrate. In 2016, 35.9% of patients registered with VWD in Brazil 2,808/7811 used one or more types of procoagulant for the treatment of any bleeding.16 Surprisingly, most of the patients (52.3%) used VWF/FVIII concentrate, followed by 42.1% and 7.6%, who used tranexamic acid and DDAVP, respectively.16 This reflects the results of the present survey, in which 82.5% vs 39.0 % or 24.0 % of the participants reported the use of VWF/FVIII concentrate vs DDAVP IV or SC in the last 12 months, respectively. This suggests that patients with an indication to use DDAVP are instead using VWF/FVIII concentrate. Our data is different from that in Windyga et al., who reported the results of a European survey conducted by The European Haemophilia Therapy Strategy Board (EHTSB). They assessed hematologists of centers across Europe, regarding clinical use of DDAVP in VWD patients, and showed that 61.5% and 53.8% of hematologists used DDAVP and VWF/FVIII concentrates, respectively, as prophylaxis for surgery in VWD.14 In this European survey, only one out of 13 participants who completed the survey did not have access to DDAVP for the treatment of VWD, due to unlicensed DDAVP in Slovakia, and 85% of the hematologists performed a DDAVP test in a non-bleeding state.14
Despite the underuse of DDAVP in Brazil, most participants showed a good general knowledge of the indications and contraindications of the drug, although some contraindications were less recognized, such as the use in coronary artery diseases. Most participants recognized the appropriate use of DDAVP as a test to evaluate responsiveness and to treat responsive VWD type 1. Physicians who recognized DDAVP as an indication for VWD Type 1 more often had prescribed DDAVP in last year (p = 0.03). However, fewer participants (about half) considered the indication of DDAVP for responsive VWD type 2 and this should be a target for education. Concerning contraindications, most participants indicated correctly that “hyponatremia, seizures, hypertension and children under 3 years” and “VWD type 2 with thrombocytopenia” are contraindications for DDAVP use. But only 42.5% considered “Coronary artery disease” as a contraindication for DDAVP use, compared with 80% of participants in the European survey who recognized coronary disease as a contraindication14. In our study, more than one-third (35.9%) of the participants considered pregnancy as a contraindication for DDAVP use. This likely reflects the controversies about the use of DDAVP before labor due to concern about the potential risk of placental insufficiency related to arterial vasoconstriction and the increase in the risk of miscarriage due to an oxytocic effect and to maternal and/or neonatal hyponatremia.20,21 This explains why some experts recommend the use of DDAVP only after the clamping of the umbilical cord.20,21 Regarding age, it is general practice to avoid DDAVP in children under three years, but there is some controversy about DDAVP use in the elderly, with variability in the recommendations.14,22 In Brazil, the MoH recommends avoidance of DDAVP in children under the age of three.22
This study poses some limitations. We only assessed participants who attended the congress instead of assessing medical doctors treating VWD in the entire country and it is to be expected that medical doctors attending a congress be more likely to be updated on medical subjects. Furthermore, most of the participants worked in southeastern Brazil, which is the most developed region of the country. Therefore, this impairs the generalizability of our study. Finally, we did not include in the survey a question inquiring whether participants currently use VWF/FVIII concentrates instead of DDAVP in clinical situations, such as major surgery, children < 3 years of age, elderly patients with a history of heart disease, in which DDAVP is contraindicated.
ConclusionIn conclusion, we showed that DDAVP is currently underused in Brazil, as opposed to the excessive use of VWF/FVIII concentrates in VWD patients. The reasons for not using it were varied and mainly included lack of reagents, laboratory facilities and of skills in using DDAVP. Participants are well-acquainted with the indications and contraindications of DDAVP. We suggest that measures targeting the adoption of communication strategies, as well as an educational program and the availability of laboratory reagents, are key to increment the correct use of DDAVP.
FundingThis study was partially funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) which included the Doctorate degree of MSSNL.
We thank all the participants of this survey for their contribution to this study. We also thank Stela Brener Verthenco for helping with the statistical analysis.
MSSNL and SMR designed the study, analyzed the data and wrote the paper.