Diffuse large B-cell lymphomas (DLBCL) comprise a group of heterogeneous neoplasms being the most frequent subtype of non-Hodgkin Lymphoma (NHL). Seven to 10% of DLBCL, most of them derived from germinal center cells, harbored cMYC, BLC2 and/or BCL6 translocations, known as “Double-hit” lymphoma (DHL) and/or “Triple-hit” lymphoma (THL). They are categorized as a high-grade B-cell lymphoma (HGBL) according to World Health Organization (WHO) classification, have an inferior prognosis and a special therapeutic approach.1,2,3 The standard technique for diagnosis of double or triple hit NHL is the fluorescence in situ hybridization (FISH) performed in paraffin-embedded tissue, using probes to detect cMYC (located in chromosome 8q), BCL2 (located in chromosome 18q) and BCL6 (located in chromosome 3q) rearrangements. Although karyotype is important to determine significant abnormalities for characterization of NHL genetic subtypes, it is not used in clinical routine, because of the difficulty to send fresh material to the laboratory. This fresh material and methanol-acetic acid-fixed cell suspensions (arising from the karyotype preparation) could also be used for performing FISH.
This study aimed to compare the results of karyotype and the cMYC, BCL2 and BCL6 rearrangements detected by FISH performed in paraffin-embedded tissues and methanol-acetic acid fixed cells suspensions in lymph nodes samples with suspicious of NHL diagnosis.
For karyotyping, cells from lymph nodes samples were obtained after mechanical disaggregation, cultured for 24-hrs without mitogen and 72-hrs cultures with phorbol-12-myristate 13-acetate (TPA), harvested and submitted to G-banding according to standard protocols.4 We use the same probes cMYC, BCL2, BCL6 (Break-apart, DC, Cytocell®, Cambridge, UK) for FISH studies on paraffin-embedded tissues fixed in 10% buffered formalin and on methanol-acetic acid (3:1) fixed cells. In some cases, other probes were used to confirm the karyotype findings, for example, FISH for detection the t(11;14) CCND1-IGH rearrangement. Images were captured with a Zeiss Axio Scope.A1 microscope and were evaluated with IKAROS and ISIS imaging system (MetaSystems, Altlussheim, Germany). FISH studies were performed according to the manufacturer’s recommendations, in 2 μm thick section paraffin block or suspension cells. A hundred cells were manually scored in each case by two biologists and reviewed by expert hematopathologists. Cut-off values were established in our laboratory following international guidelines.5cMYC, BCL2, BCL6 rearrangements cut-offs in paraffin-embedded tissue were 3.8%, 2.3% and 3.8% respectively, and in suspension cells were 11.9%, 4.6% and 6.0%. The cytogenetic results agreed with the International System for Human Cytogenetic Nomenclature 2016.6
Fresh and paraffin-embedded tissues from 4 lymph nodes were used in this study (2 diffuse large B-cell lymphomas, 1 mantle cell lymphoma, and 1 metastatic carcinoma).
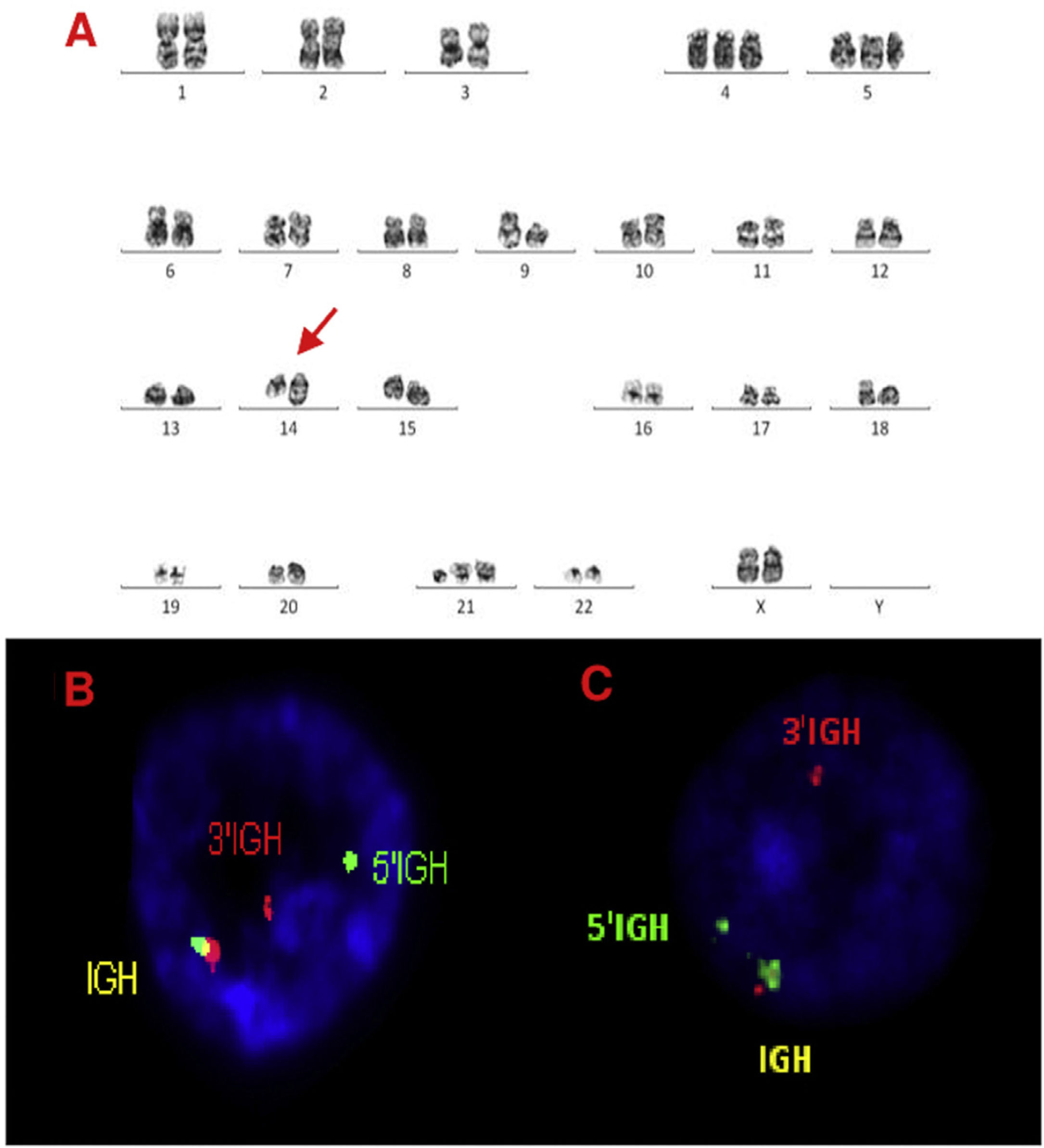
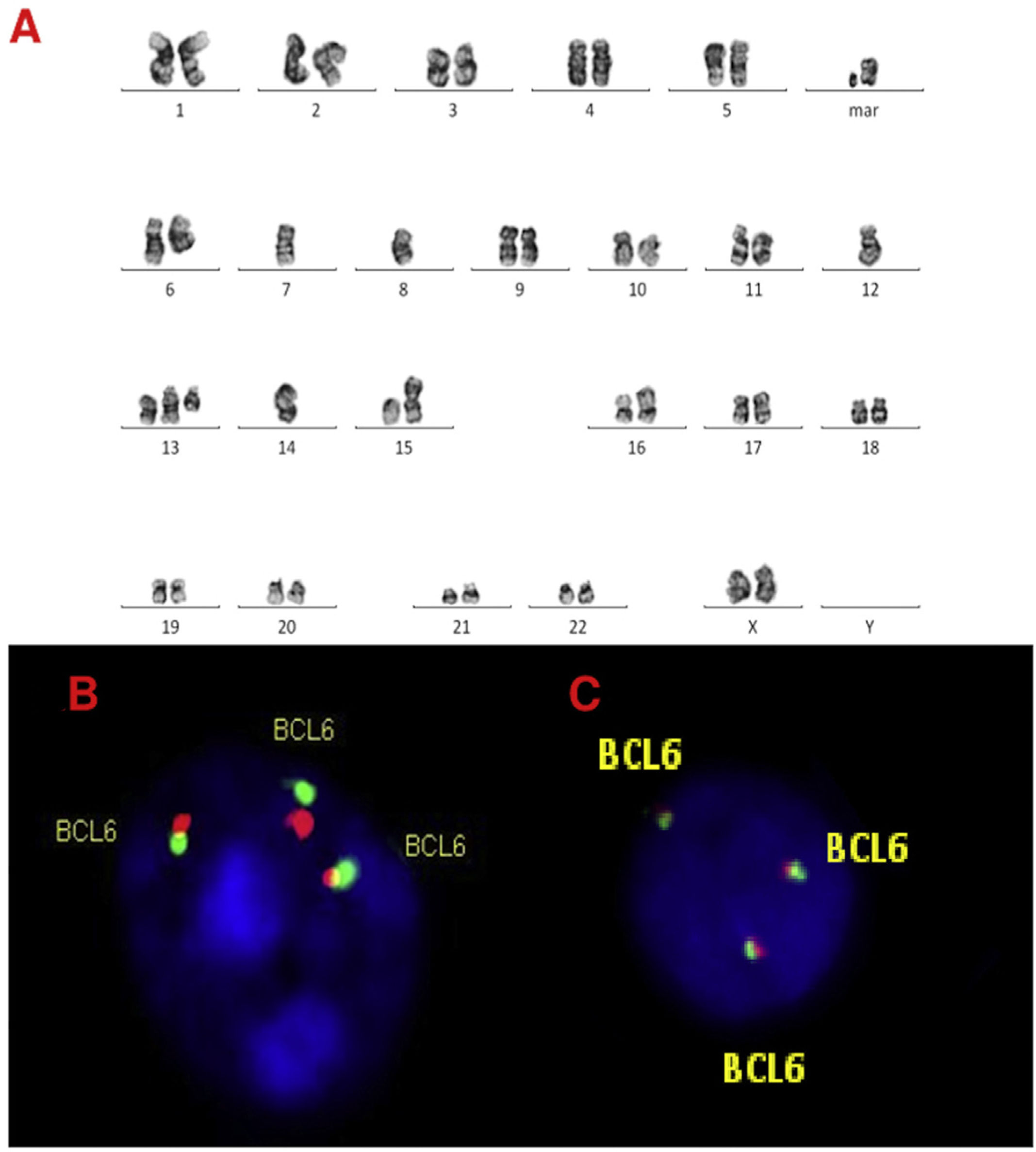
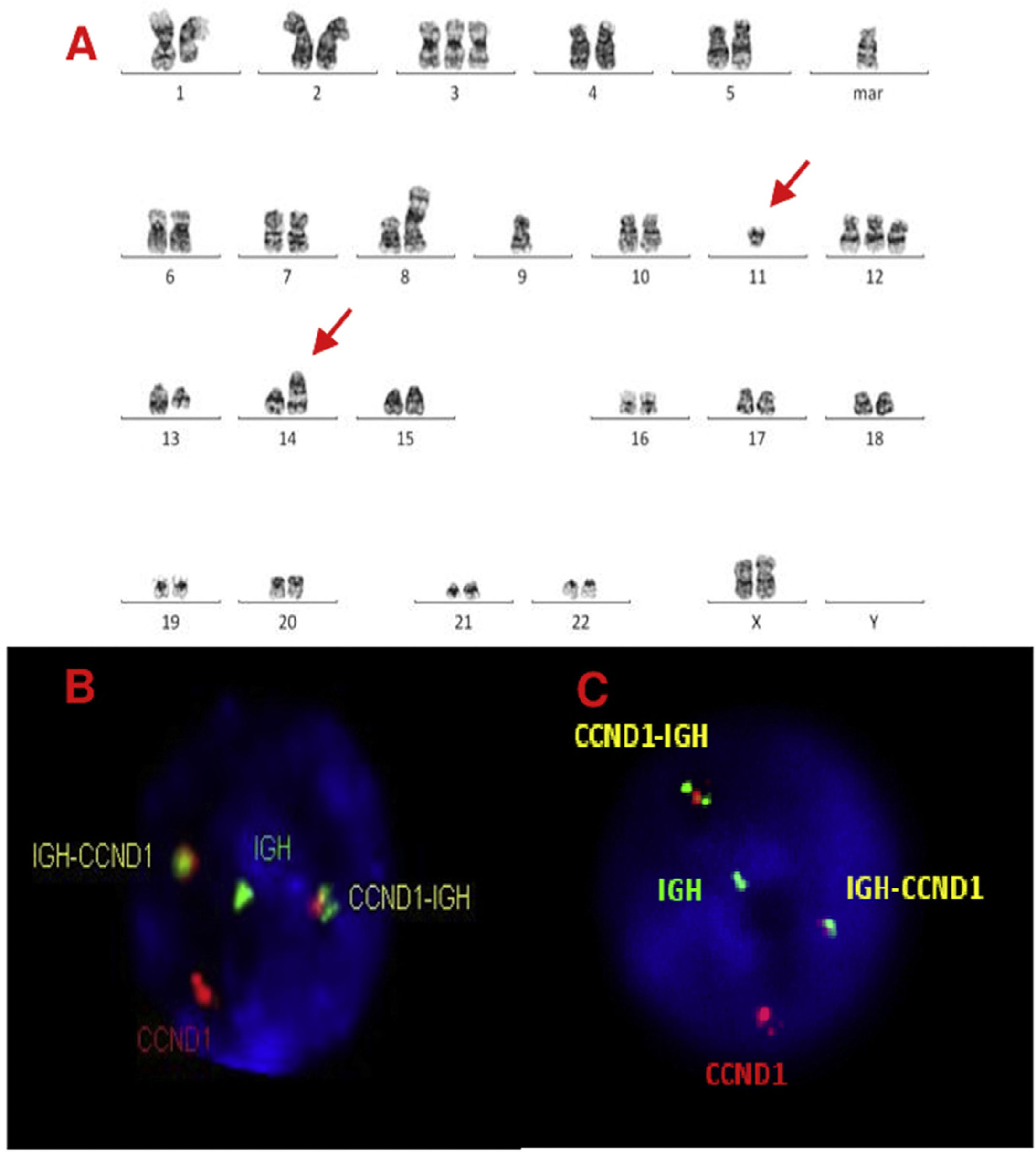
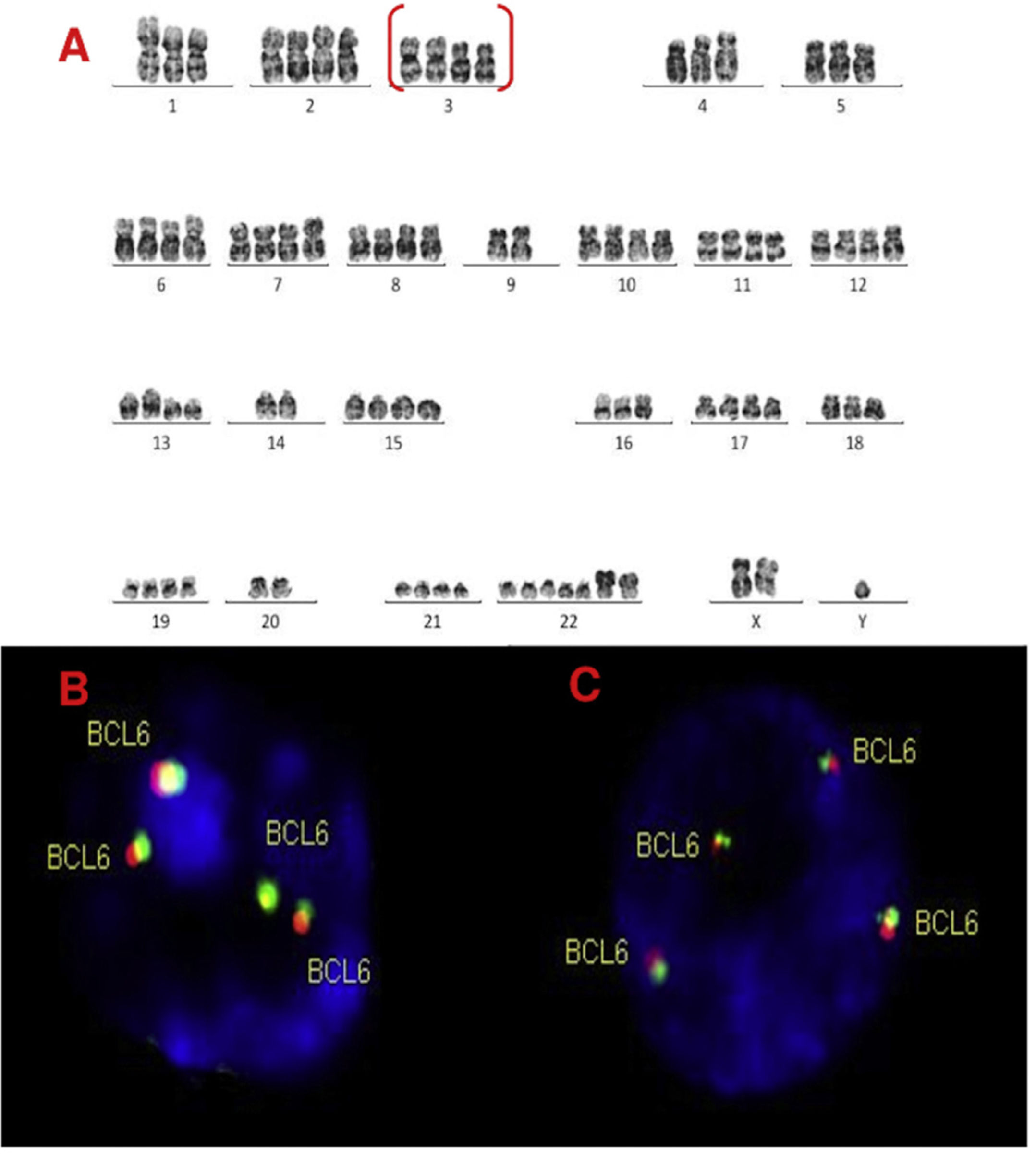
Karyotype and FISH results are shown in Table 1 and Figure 1. All karyotypes were complex, showing IGH rearrangement in 2 cases (cases 1 and 2), one of them with t(11;14) CCND1/IGH. FISH for detection of t(11;14) (CCND1/IGH DC, DF, Cytocell®, Cambridge, UK) or IGH rearrangement (IGH, Breakapart, DC, Cytocell®, Cambridge, UK) was studied in paraffin and fixed material and confirmed the results. cMYC, BCL2 and BCL6 rearrangements were not detected in paraffin-embedded tissue nor suspension cells. Extra copies of BCL6 were detected in fixed and paraffin samples in 3 cases (cases 2, 3 and 4) (Figures. 2–4).
Results of interphasic FISH for cMYC, BCL2 and BCL6 in paraffin-embedded tissue and in suspension cells and the karyotype in 4 lymph nodes samples. The numbers in parentheses represent the percentage of interphases with the cytogenetic abnormality.
| FISH paraffin | FISH suspension | Karyotype | ||
|---|---|---|---|---|
| Case 01Diffuse large B-cell lymphoma | cMYC | Negative | Negative | 49,X,add(X)(q24),der(1)dup(1)(q21q4)del(1)(q42),+4,+5,del(9)(p13),add(14)(q32),+21,add(21)(p11.2)x2[3]/46,XX[4] |
| BCL2 | Negative | Negative | ||
| BCL6 | Negative | Negative | ||
| IGH | Rearrangement (54%) | Rearrangement (56%) | ||
| Case 02Diffuse large B-cell lymphoma | cMYC | Negative | Negative | 45,XX,-7,-8,-12,add(12)(q24.1),+del(13)(q12q22),add(13)(q34),-14,t(14;?)(q32;?),add(15)(p11.1),add(16)(p13.3)+2mar[21] /46,XX[3] |
| BCL2 | Negative | Negative | ||
| BCL6 | Negative, 3 signals (46%) | Negative, 3 signals (93%) | ||
| IGH/CCND1 | Negative | Negativea | ||
| Case 03Mantle cell lymphoma | cMYC | Negative | Negative | 47,XX,add(1)(q21),+3,der(8)t(8;11;?)(p21;q13;?),-9,-11,t(11;14)(q13;q32),+12,del(13)(q12q21),+mar[8]/46,XX[12] |
| BCL2 | Negative | Negative | ||
| BCL6 | Negative, 3 signals (19%) | Negative, 3 signals (46%) | ||
| IGH/CCND1 | Present (15%) | Present (89%) | ||
| Case 04Metastatic carcinoma | cMYC | – | – | 76∼85,XXYY,-1,inv(2)(q12q24)x2,del(3)(p24)x2,-4,-4,-9,-9,del(11)(q22q25)x2,del(13)(q12q32)x2,-14,-14,-16,-20,-20,+22,+add(22)(p11.2)x2[12]/46,XY[8] |
| BCL2 | – | – | ||
| BCL6 | Negative, 4 signals (10%) | Negative, 4 signals (78%) | ||
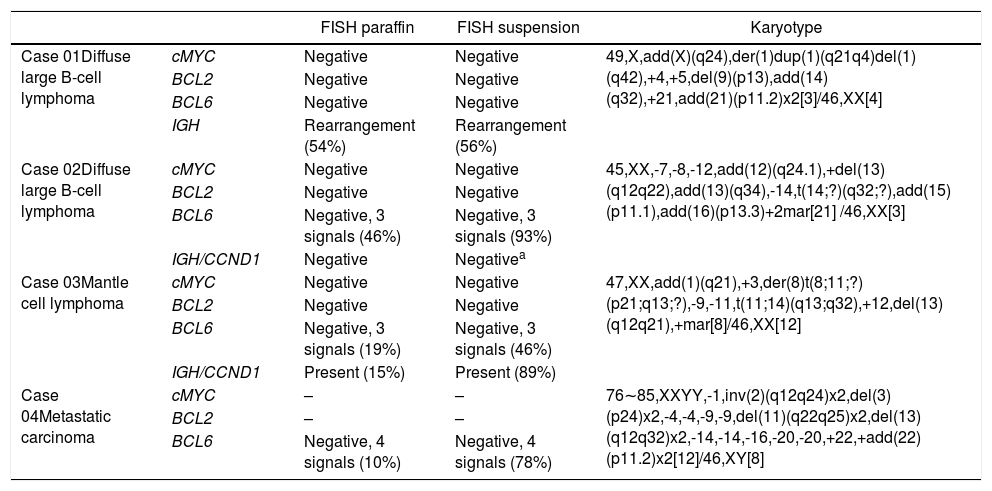
A, B and C–Cytogenetic studies from case 1: (1A) Lymph node karyotype analysis with G-banding demonstrating complex karyotype, the arrow shows additional material in 14q, (1B) interphase break-apart FISH showing IGH rearrangement, with separation of the 5′-probe (green) from the 3′-probe (red) in paraffin-embedded tissue and (1C) in suspension cells.
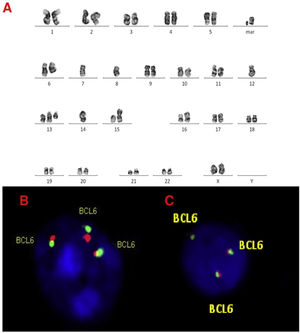
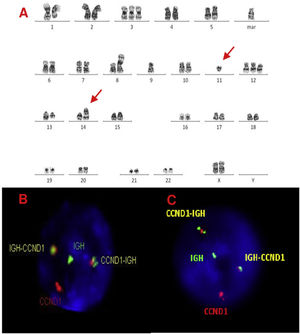
A, B and C–Cytogenetic studies from case 3: (3A) Lymph node karyotype analysis with G-banding demonstrating complex karyotype, the arrows shows t(11;14), (3B) interphase dual color, dual fusion FISH showing CCND1-IGH rearrangement, with 2 fusion signals detected in paraffin-embedded tissue and (3C) in suspension cells.
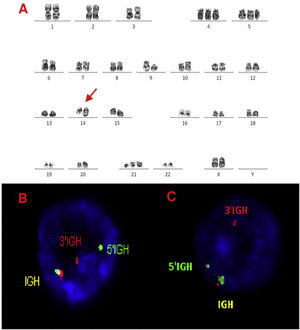
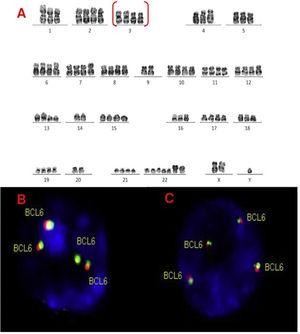
A, B and C–Cytogenetic studies from case 4: (4A) Lymph node karyotype analysis with G-banding demonstrating complex and hypotetraploidy karyotype, (4B) interphase break-apart FISH showing two extra copies of BCL6 (4 fusion signals) detected in paraffin-embedded tissue and (4C) in suspension cells.
Although we were unable to obtain double and/or triple-hit NHL in these small number of samples to compare these rearrangements in suspension cells and paraffin-embedded tissue we observed agreement for IGH rearrangement and extra BCL6 copies. We still observed a higher percentage of cells with gene rearrangements in three out of four cases in methanol-acetic acid-fixed material when compared to paraffin blocks. We had difficulty in obtaining fresh samples from biopsies in the medical routine, but we know the importance of making this material available for immunophenotypic and genetic studies, which can generate important data in a short turnaround time. We reinforce the importance of collecting fresh material during biopsies procedures in patients with NHL suspicions and challenge to perform a greater number of karyotyping and FISH studies in cell suspensions, to confirm our findings.
Conflict of interestThe authors declare no conflicts of interest.
The authors acknowledge the technical staff of the cytogenetic and the pathology laboratories for their assistance, in special to Cristine Dobo and Daniela Borri.