There have been significant improvements in therapeutic options for relapsed multiple myeloma (MM) over the past two decades, with many novel agents including proteasome inhibitors, immunomodulatory agents, and more recently monoclonal antibodies demonstrating efficacy in this setting. However, there is a paucity of real-world data comparing outcomes seen in patients treated with novel agents as opposed to older agents. We report a historical single center cohort of patients diagnosed with myeloma between the years 1991–2012 in order to explore possible differences in outcomes. A total of 139 patients who underwent stem cell transplantation were included in our study. In our study, 88 patients were treated with cyclophosphamide and steroids alone at relapse whereas 51 patients were treated with Len-Dex. In the multivariate analysis, TTNT was shorter for patients who received Cyclo compared to Len-Dex (HR=1.74; 95% CI, 1.01–2.99; p=0.04); however, we could not detect an overall survival benefit (HR=1.20; 95% CI 0.63–2.29; p=0.57). Adverse event rates were similar in the two groups. In this retrospective single center analysis, Len-Dex was associated with longer TTNT compared with Cyclo at first relapse following autoSCT in MM; however its effect on overall survival in this setting was less clear.
There has been tremendous progress in the development of treatments for multiple myeloma (MM), with nine novel agents approved by the Federal Drug Administration over the last fifteen years.1 These novel therapies include proteasome inhibitors (bortezomib, carfilzomib and ixazomib), immunomodulatory drugs (lenalidomide, pomalidomide), and anti-CD38 monoclonal antibodies (daratumumab). In many cases, the efficacy of these agents is improved when they are used in combination.2–4 The introduction of these novel therapies has correlated with recent improving survival trends for MM patients.5–8
However, the novel therapies come at a significant cost to private payers and health care authorities as treatment regimens have become increasingly expensive, with the newest agents priced as high as $10,000 USD per month.9 These costs are further magnified when multiple novel agents are used simultaneously.
In contrast to the novel agents, many older therapies for MM are relatively inexpensive. Cyclophosphamide is an effective anti-myeloma agent which has been used to treat MM patients for over 40 years.10,11 The drug costs associated with cyclophosphamide are significantly lower than with newer therapies. For example, for the average patient, the cost of cyclophosphamide in the CyBorD regimen (cyclophosphamide, bortezomib, and dexamethasone) is approximately $134 USD per cycle in the United States,12 and is considerably lower in other jurisdictions.13,14
A substantial fraction of MM patients are deemed eligible for autologous stem cell transplantation (autoSCT). This generates a significant overall survival benefit,15–17 but is not curative, and patients eventually relapse. The sequencing and combination of subsequent therapies following relapse is controversial. At our center, the preferred practice prior to the advent of the novel therapies was to treat patients who had relapsed following an autoSCT with a combination of cyclophosphamide and a glucocorticoid. This practice continued until the early 2010s, at which time the combination of lenalidomide and dexamethasone18 became the preferred therapy. To our knowledge, the relative efficacy of these two regimens in patients with relapsed or refractory MM has never been compared. We therefore sought to compare the efficacy and safety of these two regimens using historical cohorts from our center.
MethodsWe performed a retrospective observational cohort study of patients treated for MM at a single academic center between January 1, 1991 and November 6, 2016. We screened all patients who received an autoSCT, relapsed, and subsequently received at least one further line of treatment. Patients were included in the analysis if they received either cyclophosphamide with a corticosteroid (Cyclo), or lenalidomide with dexamethasone (Len-Dex) as treatment at first relapse. Patients were excluded if they had received other treatment regimens at first relapse, including bortezomib-based therapy, steroid monotherapy, or therapy with other novel agents.
Our primary outcome was time to next treatment (TTNT), defined as the time from the start of therapy at first relapse to the start of therapy at second relapse. Patients were censored at the date of last follow-up. Secondary outcomes included overall survival (OS), defined as time from diagnosis of MM to death from any cause, as well as a landmark analysis of survival from the start of therapy at first relapse to death from any cause. We also compared rates of selected adverse events (AEs) of Grade 3 or higher, as defined by the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.19 The adverse events that were included in the analysis were: anemia, neutropenia, thrombocytopenia, diarrhea, and rash. Rates of hospitalization, infection, and packed red blood cell (pRBC) or platelet transfusion were also included in the analysis.
Outcomes were assessed by Kaplan-Meier methods and overall differences determined by logrank test. Cox-proportional hazard ratios were calculated for individual treatment groups and compared by univariate and multivariate logistic regression. The multivariate analyses controlled for a set of the most clinically relevant variables (selected a priori) that the investigators believed may impact treatment efficacy and disease outcomes. These factors included age, sex, International Staging System (ISS) stage, induction regimen used prior to autoSCT, and time between diagnosis and the start of the first treatment post-relapse.
ResultsA total of 243 patients underwent treatment for MM at first relapse post autologous transplant. Of these, 139 patients were included in the analysis, of which 88 were treated with Cyclo and 51 were treated with Len-Dex. Of the 104 patients who were excluded, 69 were treated with alternative chemotherapies (35 steroid monotherapy, 33 bortezomib and steroids, 1 vincristine-doxorubicin-dexamethasone), 15 went on to have a second stem cell transplant, 17 had insufficient data, and 3 had developed plasma cell leukemia.
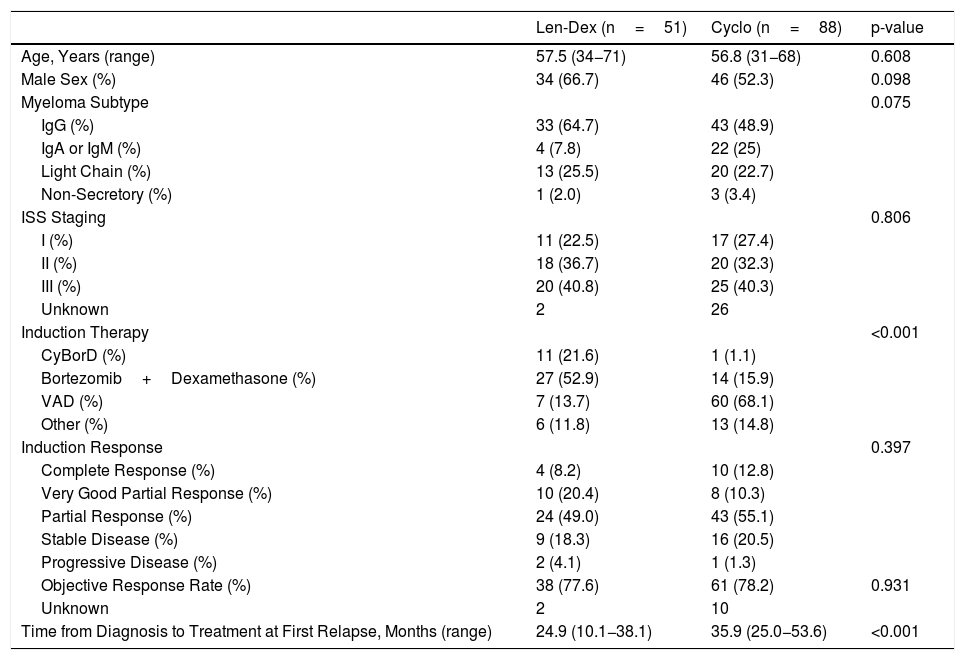
Patient demographics and disease characteristics are summarized in Table 1. Characteristics were similar between each group for age, sex, subtype of MM and ISS stage. There were significant differences between each group for induction regimen at diagnosis, and for the time from diagnosis to treatment at first relapse following autoSCT. Vincristine, doxorubicin and dexamethasone (VAD) was the most common induction treatment at diagnosis for the Cyclo group (68.1%), whereas bortezomib-based therapy was the most common for the Len-Dex group (74.5%) (p<0.001). This difference is attributable to the change in available induction regimens throughout the time period encompassed by this study. There were no significant differences in the overall response rate or the depth of response to induction. The median time from diagnosis to the time of treatment at first relapse after autoSCT was 35.9 months (Interquartile Range [IQR], 25.0−53.6 months) in the Cyclo group, and 24.9 months (IQR, 10.1–38.1 months) in the Len-Dex group, (p<0.001).
Characteristics of the Patients at Baseline.
| Len-Dex (n=51) | Cyclo (n=88) | p-value | |
|---|---|---|---|
| Age, Years (range) | 57.5 (34−71) | 56.8 (31−68) | 0.608 |
| Male Sex (%) | 34 (66.7) | 46 (52.3) | 0.098 |
| Myeloma Subtype | 0.075 | ||
| IgG (%) | 33 (64.7) | 43 (48.9) | |
| IgA or IgM (%) | 4 (7.8) | 22 (25) | |
| Light Chain (%) | 13 (25.5) | 20 (22.7) | |
| Non-Secretory (%) | 1 (2.0) | 3 (3.4) | |
| ISS Staging | 0.806 | ||
| I (%) | 11 (22.5) | 17 (27.4) | |
| II (%) | 18 (36.7) | 20 (32.3) | |
| III (%) | 20 (40.8) | 25 (40.3) | |
| Unknown | 2 | 26 | |
| Induction Therapy | <0.001 | ||
| CyBorD (%) | 11 (21.6) | 1 (1.1) | |
| Bortezomib+Dexamethasone (%) | 27 (52.9) | 14 (15.9) | |
| VAD (%) | 7 (13.7) | 60 (68.1) | |
| Other (%) | 6 (11.8) | 13 (14.8) | |
| Induction Response | 0.397 | ||
| Complete Response (%) | 4 (8.2) | 10 (12.8) | |
| Very Good Partial Response (%) | 10 (20.4) | 8 (10.3) | |
| Partial Response (%) | 24 (49.0) | 43 (55.1) | |
| Stable Disease (%) | 9 (18.3) | 16 (20.5) | |
| Progressive Disease (%) | 2 (4.1) | 1 (1.3) | |
| Objective Response Rate (%) | 38 (77.6) | 61 (78.2) | 0.931 |
| Unknown | 2 | 10 | |
| Time from Diagnosis to Treatment at First Relapse, Months (range) | 24.9 (10.1−38.1) | 35.9 (25.0−53.6) | <0.001 |
ISS denotes the International Staging System for Multiple Myeloma; CyBorD denotes cyclophosphamide-bortezomib-dexamethasone regimen; VAD denotes vincristine-doxorubicin-dexamethasone regimen.
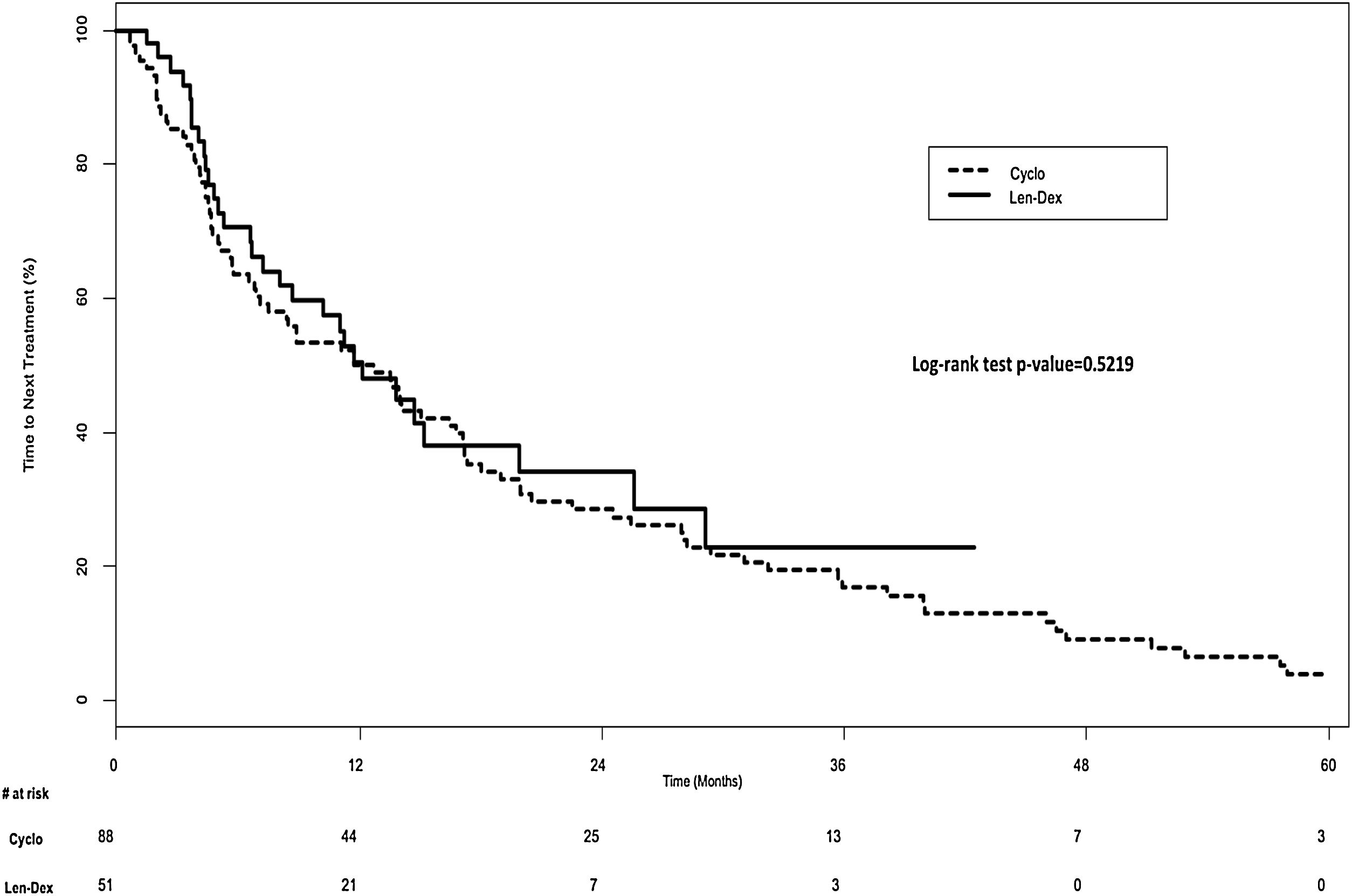
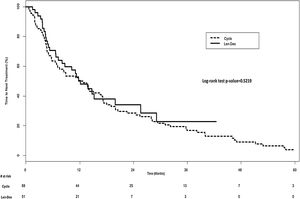
In the univariate analysis, TTNT was similar in both groups; 12.1 months (IQR, 4.8–29.2 months) in the Len-Dex group, vs. 12.2 months (IQR, 4.6-28.0 months) in the Cyclo group, p=0.52 (Figure 1). However, after adjusting for age, sex, ISS stage, induction regimen used prior to autoSCT, and time between diagnosis and the start of the first treatment post-relapse in a multivariate model, Cyclo was associated with a shorter TTNT compared with Len-Dex, (Cyclo vs. Len-Dex; Hazard Ratio [HR] 1.74; 95% Confidence Interval [CI], 1.01–2.99; p=0.04).
Results of the multivariate analysis are seen in Table 2. Patients with a longer duration from the time of diagnosis to their first relapse after autoSCT appeared to have a longer TTNT than those with a shorter duration (increase of 1 month; HR 0.98; 95% CI 0.97−0.99; p=0.002). Paradoxically, patients with ISS stage I disease appeared to have a shorter TTNT than patients with ISS stage II disease in this cohort (ISS stage I vs stage II HR 1.86; 95% CI 1.03–3.38; p=0.041).
TTNT Multivariate Analysis.
| Comparison | Hazard Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|---|
| Cyclo vs Len-Dex | 1.74 | 1.01 | 2.99 | 0.044 |
| Increase in age by 1 year | 1.00 | 0.97 | 1.02 | 0.768 |
| Female vs Male | 1.53 | 0.98 | 2.39 | 0.060 |
| I vs III ISS Stage | 1.12 | 0.63 | 1.97 | 0.701 |
| II vs III ISS Stage | 0.60 | 0.36 | 1.01 | 0.056 |
| I vs II ISS Stage | 1.86 | 1.03 | 3.38 | 0.041 |
| Bortezomib-based vs. VAD induction regimen | 0.75 | 0.39 | 1.41 | 0.364 |
| Bortezomib-based vs. other induction regimen | 0.98 | 0.47 | 2.03 | 0.962 |
| Other vs. VAD induction regimen | 0.76 | 0.43 | 1.35 | 0.349 |
| Increase in time from diagnosis to treatment at first relapse by 1 month | 0.98 | 0.97 | 0.99 | 0.002 |
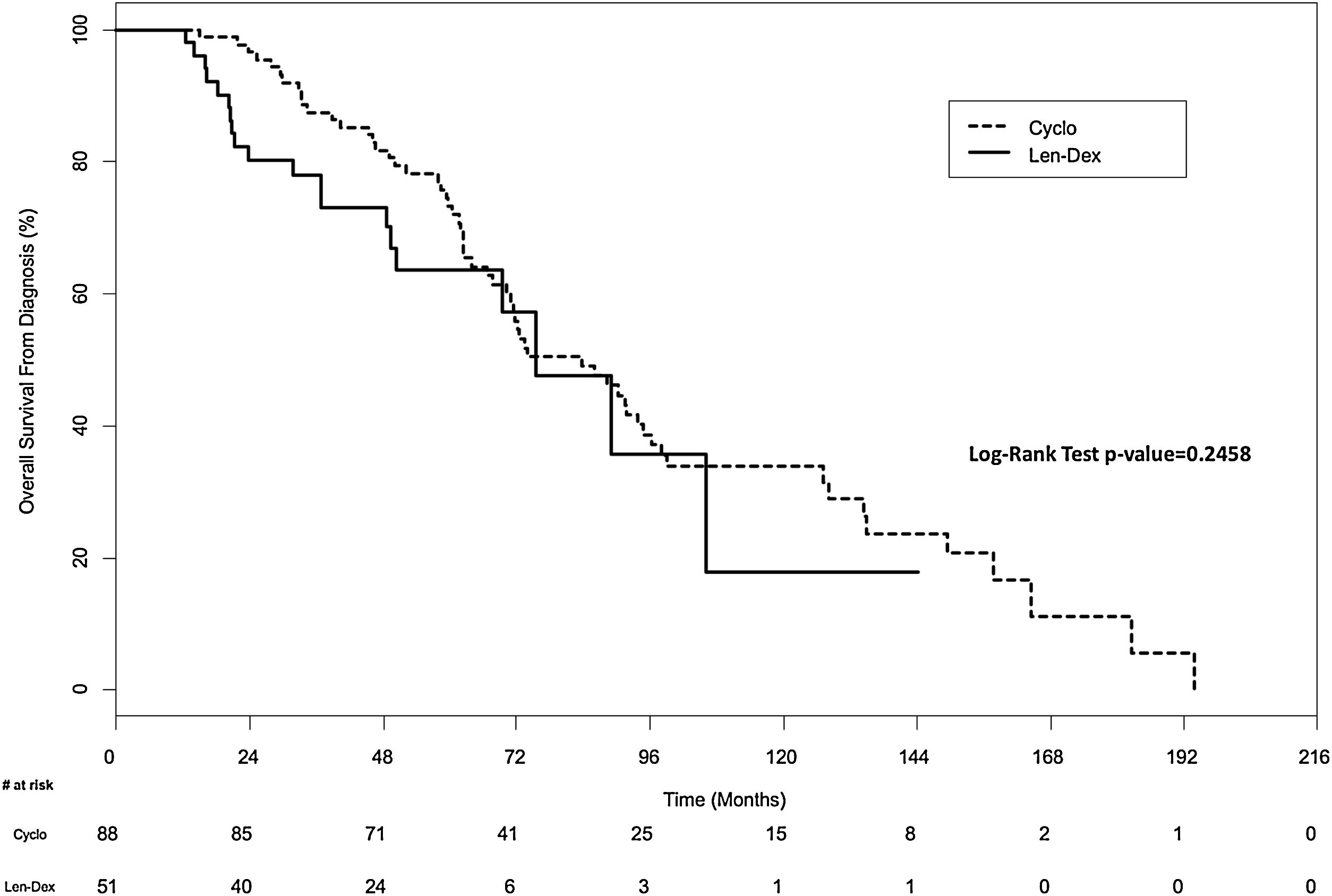
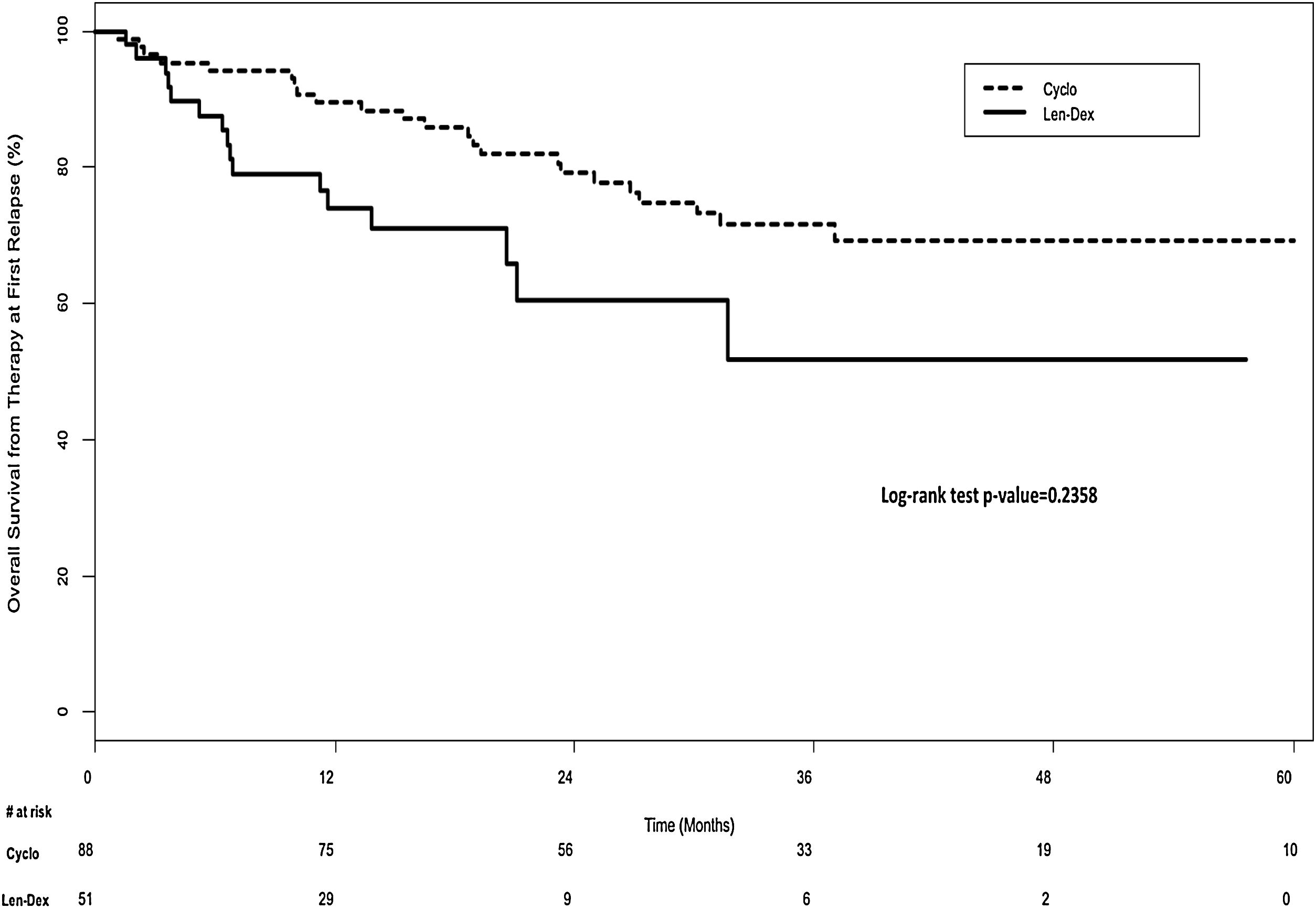
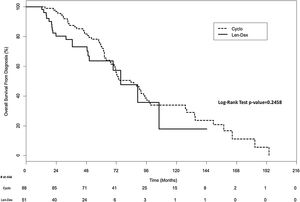
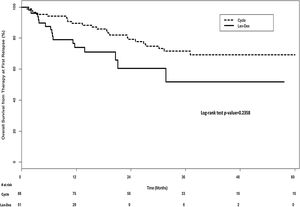
There was no significant difference in overall survival between the two groups. In the univariate analysis, the median overall survival from diagnosis was 83.6 months (IQR, 59.3–134.9 months) for the Cyclo group and 75.5 months (IQR, 36.8–106.1 months) for the Len-Dex group (p=0.25) (Figure 2). In the multivariate analysis, the HR for overall survival from diagnosis for Cyclo as compared to Len-Dex was 1.20 (95% CI 0.63–2.29; p=0.57). There was also no significant difference in the landmark analysis of overall survival from the time of therapy at first relapse to death from any cause. In the univariate analysis, the median overall survival from first relapse treatment was 35.8 months (IQR, 19.0–57.6 months) for the Cyclo group and 31.6 months (IQR, 11.3–50.6 months) for the Len-Dex group (p=0.24) (Figure 3). In the multivariate analysis, the HR for overall survival from first relapse treatment for Cyclo as compared to Len-Dex was 0.93 (95% CI 0.49–1.77; p=0.82).
Median follow-up was 79.4 months in the Cyclo group and 34.6 months in the Len-Dex group.
In patients whose responses could be evaluated, ≥VGPR responses were seen in 10 of 73 patients (14%) in the Cyclo group and 21/48 patients (44%) in the Len-Dex group.
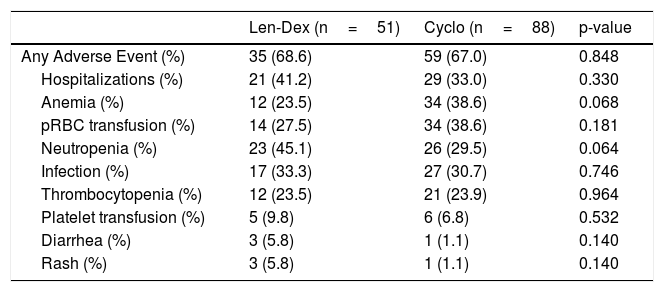
Finally, rates of grade 3 adverse events, transfusions, infections, and hospitalizations, were all similar between the two groups (Table 3).
Adverse Events.
| Len-Dex (n=51) | Cyclo (n=88) | p-value | |
|---|---|---|---|
| Any Adverse Event (%) | 35 (68.6) | 59 (67.0) | 0.848 |
| Hospitalizations (%) | 21 (41.2) | 29 (33.0) | 0.330 |
| Anemia (%) | 12 (23.5) | 34 (38.6) | 0.068 |
| pRBC transfusion (%) | 14 (27.5) | 34 (38.6) | 0.181 |
| Neutropenia (%) | 23 (45.1) | 26 (29.5) | 0.064 |
| Infection (%) | 17 (33.3) | 27 (30.7) | 0.746 |
| Thrombocytopenia (%) | 12 (23.5) | 21 (23.9) | 0.964 |
| Platelet transfusion (%) | 5 (9.8) | 6 (6.8) | 0.532 |
| Diarrhea (%) | 3 (5.8) | 1 (1.1) | 0.140 |
| Rash (%) | 3 (5.8) | 1 (1.1) | 0.140 |
Recorded instances of Anemia, Neutropenia, Thrombocytopenia, Diarrhea, and Rash represent events of severity of Grade 3 or higher as defined by the Common Terminology Criteria for Adverse Events v4.03. Events shown in the table above occurred in ≥5% of patients in either group.
To our knowledge, this is the first study to compare Len-Dex to Cyclo treatment strategies for relapsed MM following autoSCT. We found that Len-Dex resulted in a significantly longer TTNT than Cyclo. There was no significant difference in overall survival from diagnosis, landmark analysis of overall survival from first relapse treatment, and adverse event rates between the groups.
Our findings were similar to what was seen in the FOCUS trial, which randomized 315 patients with relapsed MM to single-agent carfilzomib vs. low dose corticosteroids with optional cyclophosphamide (95% of patients received the cyclophosphamide). The primary endpoint of the FOCUS trial was OS, which was similar between the two groups, with median OS of 10.2 months in the carfilzomib group and 10.0 months in the control arm (HR 0.975; 95% CI 0.760–1.249; p=0.4172). Progression free survival was also similar between the two groups. The authors noted that the patients in the control arm performed better than expected, and suggested that further evaluation of the control arm in the setting of relapsed MM was warranted.20 The results of the FOCUS trial suggest that cyclophosphamide has activity even in heavily pre-treated patients.
In our study, TTNT was highly dependent on the duration of first line therapy including autoSCT. This supports the notion that a patient’s tendency to respond to treatment is an important prognostic factor in addition to the specific choice of treatment the patient receives – in other words, patients with favorable disease biology tend to have longer remissions. This is an important consideration given the relative wealth of treatment choices in today’s myeloma landscape, and supports a risk or biology adapted approach to therapy.
We were surprised to see a significant difference in the time from diagnosis to treatment at first relapse, which was longer in the Cyclo group. We suspect this is due to historical differences in treatment availability, as most of the patients in the Cyclo group were treated during an era when there were a relatively limited number of therapeutic options available for MM patients, and when clinicians demonstrated a greater tendency to defer treatment until patients developed evidence of overt clinical progression. In the modern era, clinicians are inclined to initiate treatment at earlier stages of biochemical progression. Interestingly, the time to treatment at first relapse was longer in the Cyclo group, which may have led to less favorable outcomes and may partially explain the improved TTNT for the Len-Dex group in the adjusted analysis. It is possible that earlier treatment in the Cyclo group would have resulted in longer TTNT.
Overall survival from both diagnosis and in the landmark analysis from first relapse treatment were not different between the two groups, suggesting that both treatment regimens are active in patients with relapsed MM, and supporting the argument that disease biology plays an important role in response to treatment.
To our knowledge, this is the only study to compare the efficacy of these two treatment options for multiple myeloma at first relapse. The major strengths of our study were the use of clinically relevant endpoints, and our comparative cohort design using treatment strategies from two separate eras which eliminates the potential for treatment selection bias that would have been present had both agents been available throughout. Our study also contained a number of important limitations, most notably the small sample size, and the retrospective nature of the study which did result in differences at baseline between the two groups. Also, although we controlled for many variables, it remains possible that secular trends and differences in additional unaccounted variables may have varied between groups and affected outcomes.
We recognize that, in the developed world, standards of care in relapsed MM are changing, and that for most patients, doublet, triplet or even quadruplet therapy with novel agents has become standard of care. However, the costs of the novel agents are often prohibitive in many resource-limited settings. Our study suggests that the efficacy of cyclophosphamide-based therapy may not be substantially different from newer therapies such as Len-Dex and therefore should not be considered obsolete in managing relapsed myeloma. Further prospective comparative effectiveness studies are needed to determine the optimal and most cost-effective treatment strategy in this challenging setting.
Author contributionsRS collected data and wrote the paper. AF collected data. RM performed statistical analysis. NK and HA provided critical appraisal of the paper. AM provided critical appraisal of the paper and designed the research study.
Conflicts of interestThe authors declare no financial conflicts of interest.