The aims of this study were to identify the main characteristics regarding the shape and size of the craniofacial region in patients with sickle cell anemia (SCA) and sickle cell trait (SCT) and in unaffected patients using geometric morphometrics and to check the efficiency of this method.
Material and MethodsA cross-sectional analytical study of 45 patients (15 in each group) was performed. Lateral radiographs of the skull were used for the analysis. Seventeen landmarks and semilandmarks were placed for the measurements. The Pocrustes analysis of variance (ANOVA), regression analysis, multivariate analysis of variance, canonical variate analysis, Mahalanobis and Procrustes distances and unweighted pair group method with arithmetic mean (UPGMA) clustering were performed. Allometric effects and sex characteristics were not statistically significant (p > 0.05).
ResultsThere were, however, significant differences (p < 0.05) in craniofacial shape among SCA, SCT and unaffected individuals. Those with SCA showed variations in the shape of the external auditory meatus and at the base of the occipital bone, in addition to the mandibular setback and upper incisor inclination, with a tendency towards prognathism. The individuals with SCT exhibited a similar craniofacial shape to those with SCA, but with slighter variations. Moreover, those with SCT were statistically closer in resemblance to unaffected individuals, given that SCT is not regarded as a disease.
ConclusionThis demonstrates the efficiency of geometric morphometrics in the categorization of the assessed groups.
Sickle cell disease (SCD) is one of the most common genetic and inherited diseases worldwide. It is caused by a mutation in the gene that produces hemoglobin A, giving rise to a recessive mutant hemoglobin molecule – hemoglobin S.1 This disease has the greatest clinical significance and is characterized by the presence of atypical hemoglobin (HbS), in homozygous form (HbSS) on the one hand, and by heterozygous carriers (HbAS) of sickle cell trait (SCT) on the other hand. The individuals who received the mutant hemoglobin from one parent only (heterozygotes) are considered to be healthy.2 Since its first description in 1910, SCD has been associated with a higher prevalence among black individuals.3
The major clinical manifestation of SCD is episodic acute ischemic pain,4 whereas chronic manifestations include sickle cell nephropathy, acute chest syndrome, avascular necrosis, cerebral vascular accident, sickle cell retinopathy, delayed growth and leg ulcers, among others.5 The most common oral manifestations are mucosal pallor, delayed tooth eruption in children, smooth, discolored and depapillated tongue and cranial deformities, such as oxycephaly.6
It is recommended that dental surgeons understand the pathophysiology of sickle cell anemia (SCA) so that they can plan the treatment, taking into account the systemic manifestations of this disease.7 SCA interferes with the development of bones and teeth; therefore, the earlier the diagnosis, the sooner preventive measures can be adopted.8 Extraoral manifestations, such as convex facial profile, saddle nose, zygomatic prominence, lip incompetence, elongated face, vertical maxillary excess and maxillary atresia have been observed in SCA patients.9
One of the available methods for assessing the effect of SCA and verifying facial changes is anthropometry, which was used by Pithon et al.10 However, another method, known as geometric morphometrics, was developed in the 1980s and widely employed in the early 1990s, yielding robust analyses and making use of graphic tools.11 This method gathers and provides more information on morphology in a more efficient way than does the conventional technique.12 The data are obtained from landmarks whose points follow the rules of homology and have reliable anatomic definitions.13
In addition, geometric morphometrics assesses the variability in shape, regardless of size, using the Cartesian coordinate system, maintaining the geometric information on original data and the information on covariation between landmarks.14
Hence, even though SCA is one of the most widely investigated genetic diseases worldwide, little is known about the changes, especially those involving the craniofacial skeleton, that may occur in SCT patients. Therefore, the aims of this study were to identify the main characteristics regarding craniofacial shape and size in SCA and SCT patients, to compare them with unaffected individuals using geometric morphometrics and to check the efficiency of this method in identifying these patients and also the hypothesis that there are differences in craniofacial morphology among controls and SCA and SCT patients.
MethodologyA cross-sectional analytical study of the clinically unaffected (controls), SCT and SCA patients was performed. Forty-five patients (15 in each group) with a mean age of 20.8 years were included. The SCA average was 21 years (min. 5 and max. 56 years); the SCT average was 30 years (min. 14 and max. 53 years), and; the non-affected patients mean age was 31 years (min. 14 and max. 53 years). All study participants were enrolled in the newborn screening program. This study was approved by the Research Ethics Committee. Sample size calculations were performed. In each group, a sample size of 15 subjects was estimated at a power of 80% and a .05 level of significance, which enabled the detection of significance among the three groups.
The SCA and SCT patients were randomized at an SCA treatment center. The randomization was performed by a researcher who did not participate in the study, taking into consideration the population size and sample size for each group. A random numbers table was built using the BioEstat 5.0 (Civil Society Mamirauá, AM, Brazil). Confidentiality was ensured by using sequentially numbered brown envelopes containing the groups, following the order of the randomly drawn numbers. The controls belonged to the same community. The inclusion criteria were SCA or SCT patients diagnosed by lab tests and not subjected to orthodontic or orthognathic treatment. The patients with congenital syndromes and craniofacial anomalies, those who had undergone any type of facial surgery (bone or soft tissues) and those who were completely edentulous were excluded from the study. Each patient had a skull radiograph taken in lateral view. The radiographs were made by a radiologist using a conventional technique and the same equipment (Pax-400 Digital X-ray Imaging System, Vatech Co. Ltd., Lee, VA, USA). The images were acquired using a focal point of 0.5 mm, a 2.8-mm aluminum filter, and X-ray machines with 90 KVP and 10 mA, the latter of which was determined according to the patient’s height. There was no loss of data.
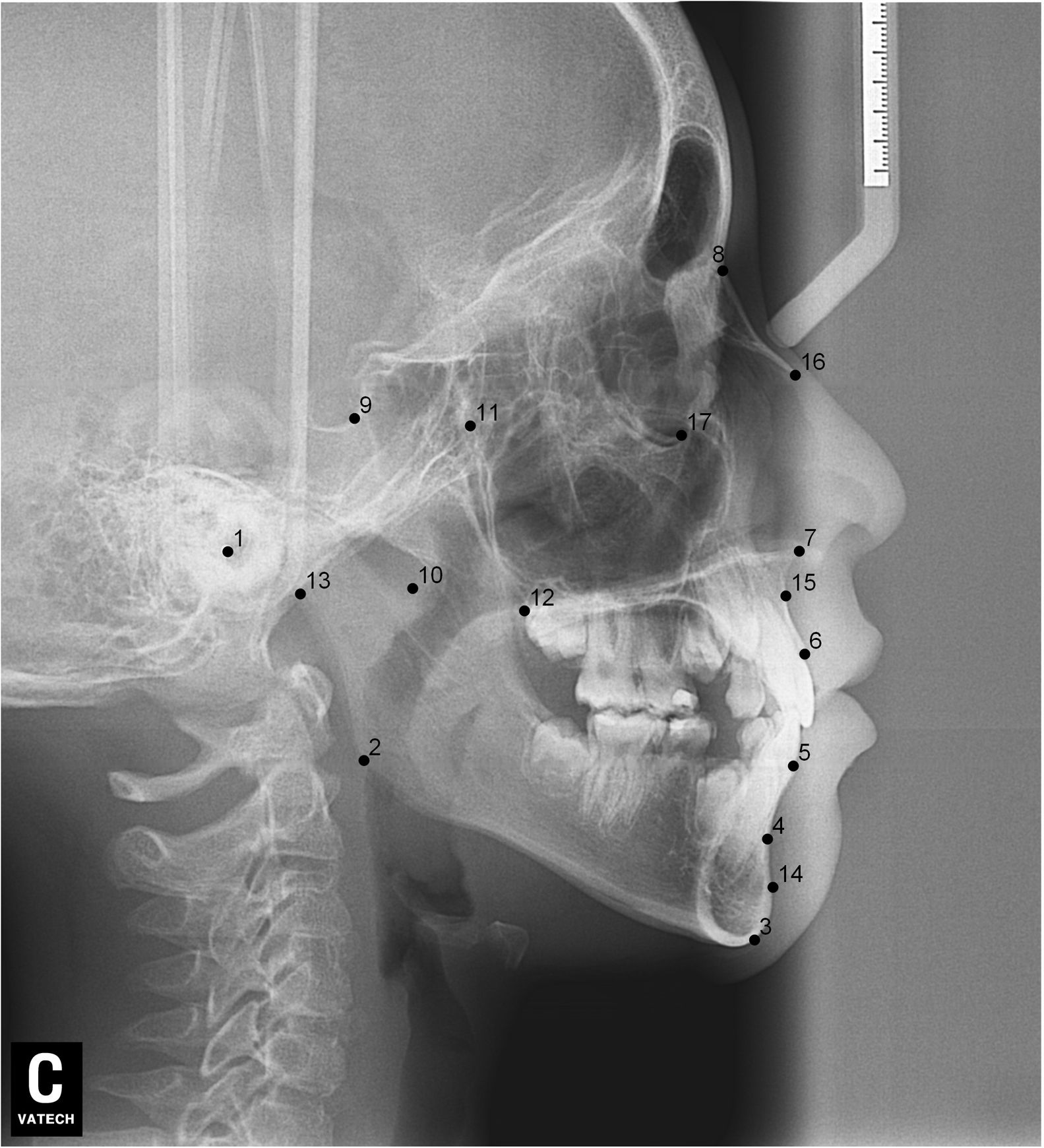
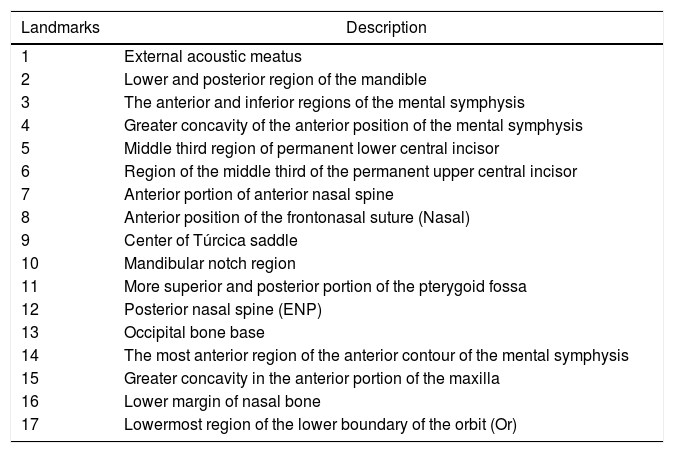
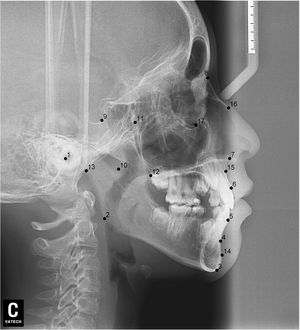
After lateral teleradiographs were taken, all images were transferred into the tpsUtil12 program, in which they were converted to the TPS format. Seventeen landmarks and semilandmarks were selected and the measurements were performed using the tpsDig215 software (Table 1, Figure 1).
Description of the landmarks used in the morphometric analyses.
| Landmarks | Description |
|---|---|
| 1 | External acoustic meatus |
| 2 | Lower and posterior region of the mandible |
| 3 | The anterior and inferior regions of the mental symphysis |
| 4 | Greater concavity of the anterior position of the mental symphysis |
| 5 | Middle third region of permanent lower central incisor |
| 6 | Region of the middle third of the permanent upper central incisor |
| 7 | Anterior portion of anterior nasal spine |
| 8 | Anterior position of the frontonasal suture (Nasal) |
| 9 | Center of Túrcica saddle |
| 10 | Mandibular notch region |
| 11 | More superior and posterior portion of the pterygoid fossa |
| 12 | Posterior nasal spine (ENP) |
| 13 | Occipital bone base |
| 14 | The most anterior region of the anterior contour of the mental symphysis |
| 15 | Greater concavity in the anterior portion of the maxilla |
| 16 | Lower margin of nasal bone |
| 17 | Lowermost region of the lower boundary of the orbit (Or) |
All markings were carried out twice by a single researcher. The Procrustes ANOVA was performed to rule out and test the effect of measurement error, as proposed by Palmer.16 Later, the semilandmarks were aligned by the tpsRelw15 software using the Procrustes least squares, turning semilandmarks into reliable landmarks, making it possible to perform the analyses. Semilandmarks allow for the quantification of the curves and concomitant analysis with the landmarks.17 Subsequently, the Procrustes superimposition was assessed based on the Cartesian coordinates generated by the landmarks. This method converts the coordinates of the original data to shape coordinates, eliminating the effects of scale, position, and direction.14
The regression analysis was performed to check the effect of allometry as a function of development (shape vs. size), owing to the different ages of the individuals analyzed. The effect of sex characteristics was evaluated as well. Multivariate analysis of variance (MANOVA), canonical variate analysis (CVA), and Mahalanobis and Procrustes distances were calculated to check the variation in the shape of the assessed structures. Both distances were used, since the Mahalanobis distance is linear and takes into account the correlation between the datasets and the scales of each axis, with the Euclidean distance correction.18 The Procrustes distance, however, is geodesic, measuring the difference in shape between two specimens, representing the natural metric in Procrustes shape space or Kendall’s shape space.17,19
Furthermore, the discriminant function and cross-validation were determined by the MorphoJ.20 The Unweighted Pair Group Method with Arithmetic mean (UPGMA) clustering and size difference analysis, based on centroid size, were estimated using the PAST software.21 The centroid size is the squared root of the sum of squared distances between all landmarks and their centroid, i.e., it is the arithmetic mean of all landmarks.13
ResultsThe regression analysis performed to evaluate the SCA, SCT, and unaffected groups showed no significant values (p > 0.05) for the effect of allometry. In other words, the shape of the assessed structures is not influenced by size. No significant differences were found between men and women (p > 0.05).
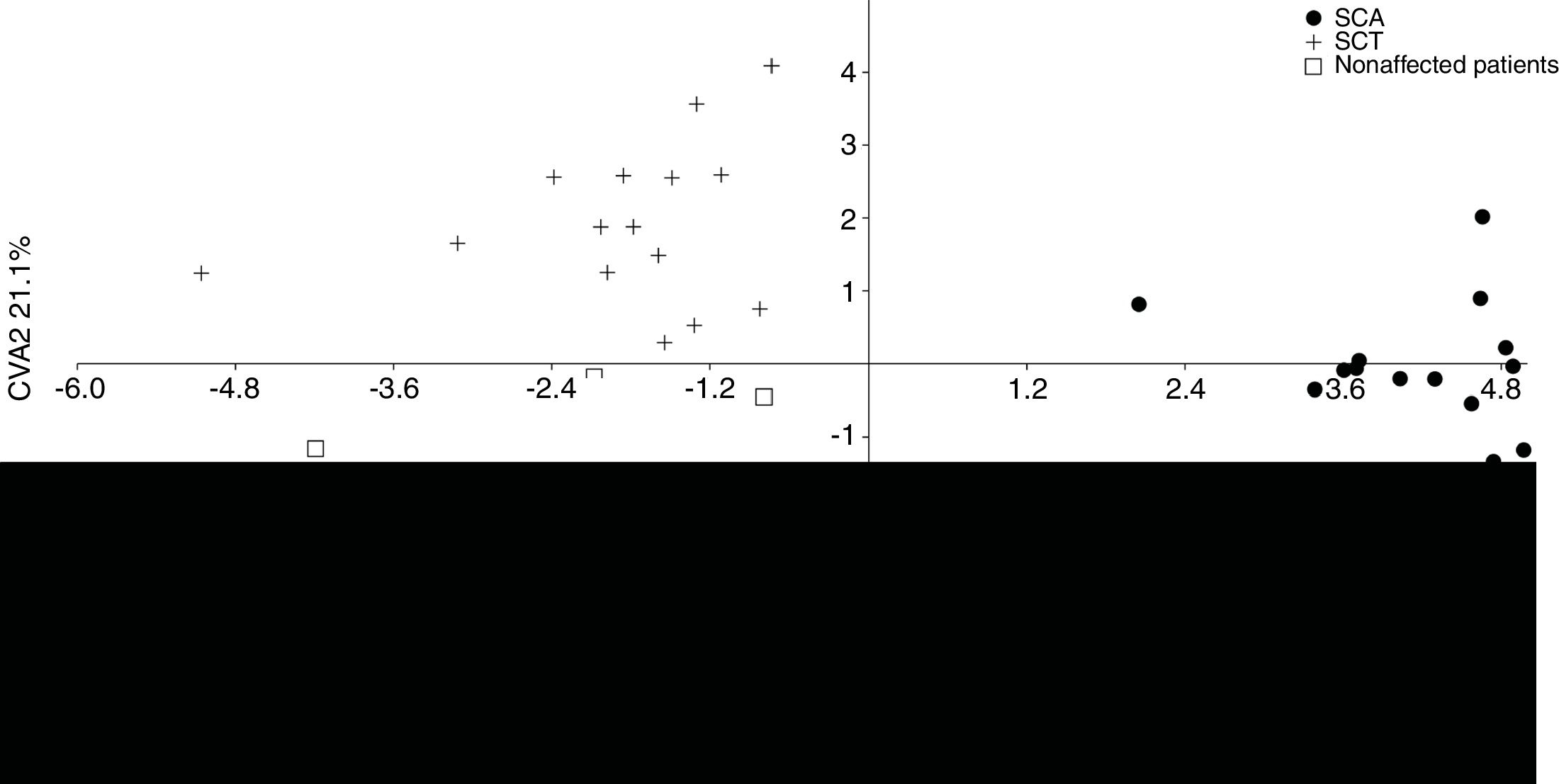
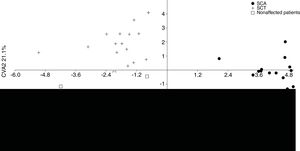
The MANOVA revealed significant values (p < 0.05), indicating differences in shape among the groups. According to the CVA, the first two canonical variables demonstrate 100% of difference in lateral view among the groups. The first one explains 78.9%, separating the SCA patients from the other groups, whereas the second one explains 21.1%, separating the SCT patients from the unaffected individuals (Figure 2).
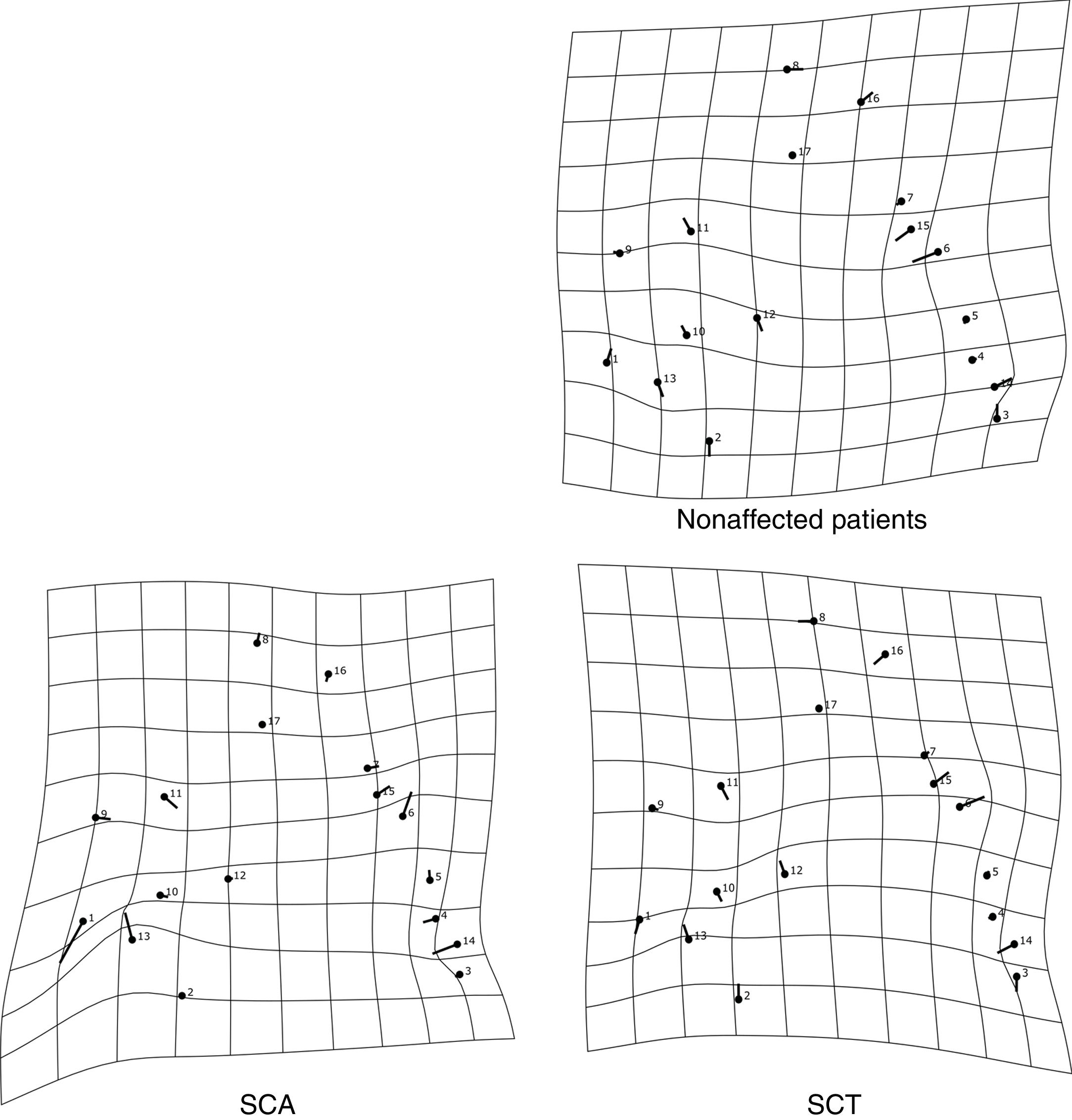
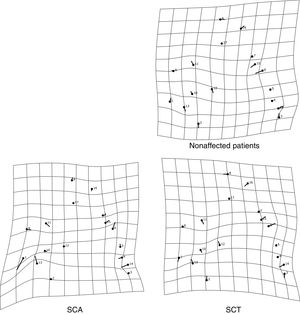
The thin-plate spline shows the differences in shape among the groups. The variation in shape in the SCA group refers especially to the variation in the external auditory meatus and at the base of the occipital bone, in addition to the mandibular setback and large upper central incisor inclination, showing a tendency towards prognathism in this group. The characteristics of the SCT group resembled those of the SCA group, but the variations were more subtle, especially the maxillary prognathism, with slighter upper central incisor inclination and mandibular setback. The control group showed different variations from those observed in the other groups, without any tendency towards mandibular setback or maxillary prognathism (Figure 3). The thin-plate spline uses an interpolation algorithm that serves as the mathematical basis for the visualization of shape differences, using images or warped surfaces.17
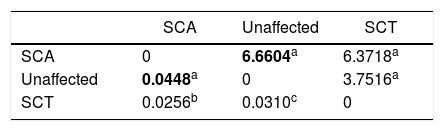
The measurements of the Mahalanobis and Procrustes distances, with at least 10,000 replications, showed that the greatest distances were between the SCA patients and the unaffected individuals (Table 2).
The discriminant function yielded significant values (p < 0.01) after 10,000 permutations. According to the cross-validation, the largest differences were found between the SCA patients and the unaffected individuals, similar to the measurements of the Mahalanobis and Procrustes distances, with 97.7% of the individuals being classified correctly according to their respective groups, followed by 70% for the SCA and SCT groups and 66% for the SCT and unaffected groups.
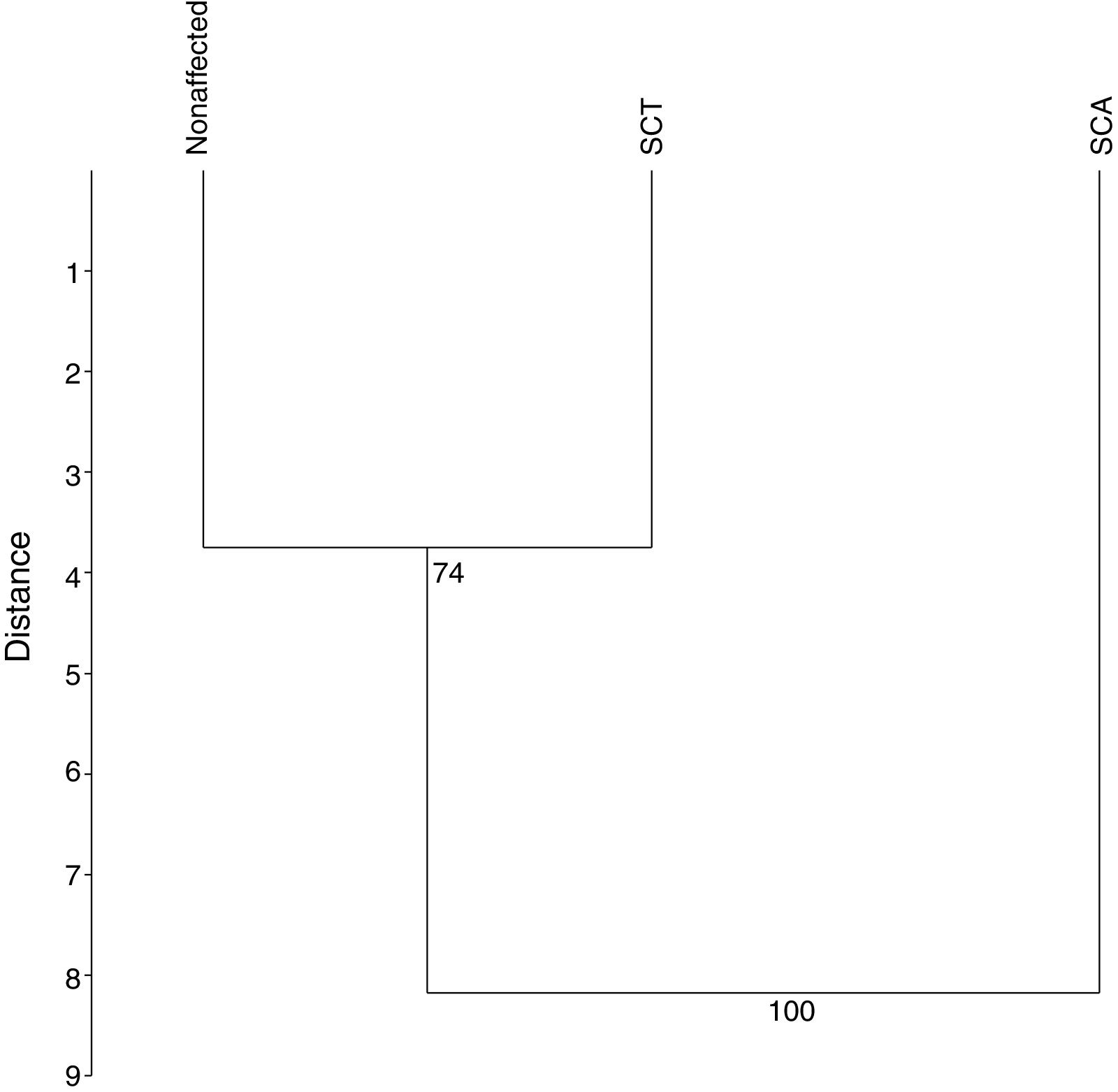
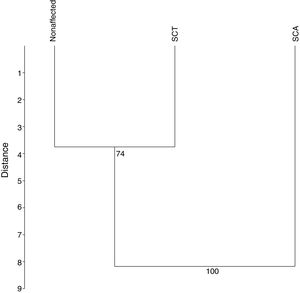
The UPGMA analysis, after 10,000 permutations and a cophenetic correlation of 93%, revealed that the SCA patients differ from those in the other groups (Figure 4), which is consistent with the findings in the previous analyces of this study.
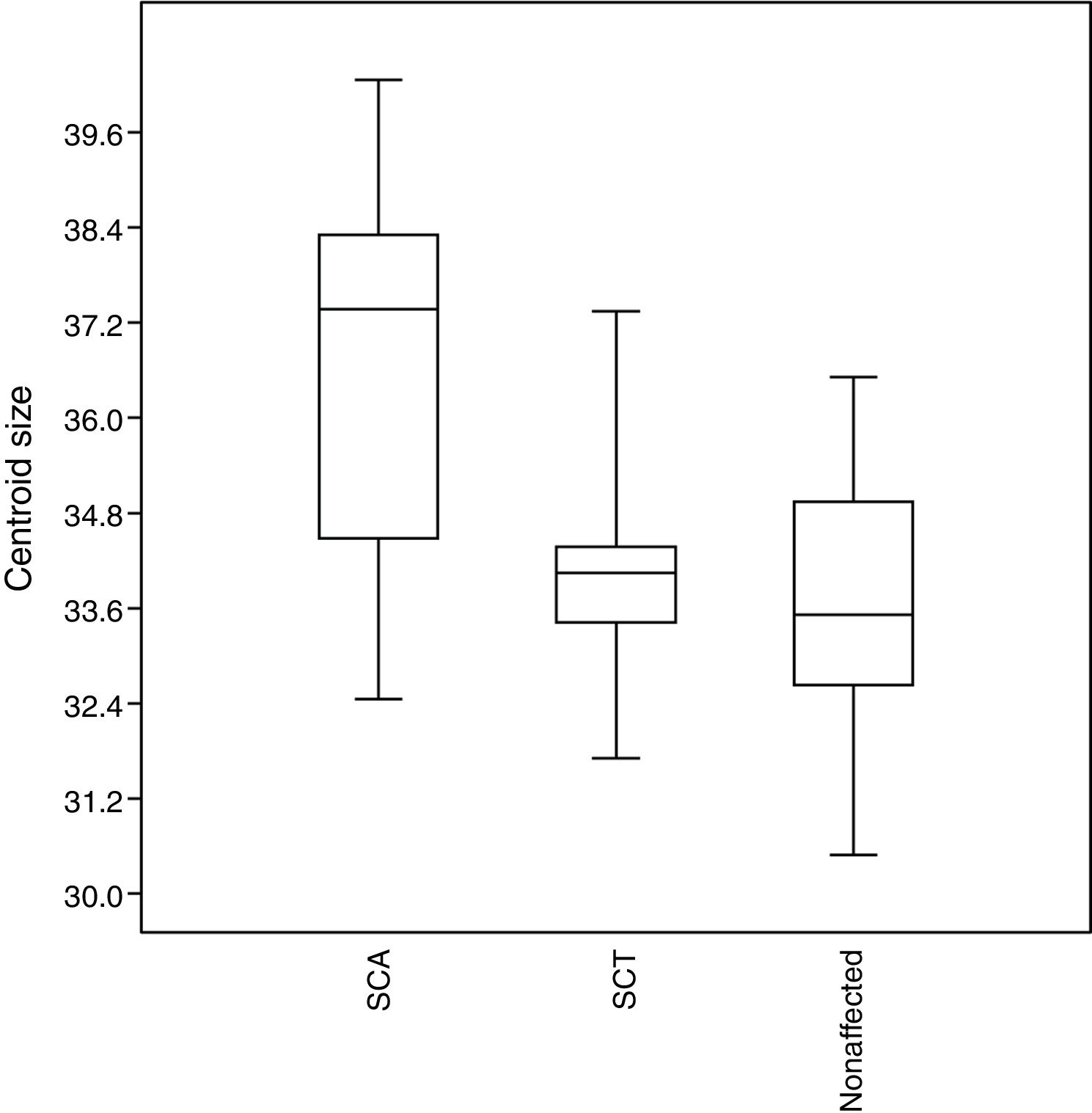
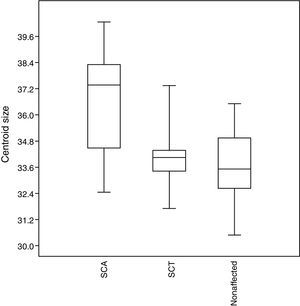
No significant results (p > 0.05) were observed for the size of the structures assessed. However, the SCA patients tend to have larger craniofacial structures than those in the other groups, whereas unaffected individuals tend to have smaller structures (Figure 5).
DiscussionThe evaluation of the differences in shape and size among SCA and SCT patients and unaffected individuals allowed for the establishment of distinctions among, and the characterization of, the groups. A study undertaken by Pithon et al.,10 with the aim of categorizing the same groups using traditional morphometrics, demonstrated no significant differences between unaffected individuals and SCT patients or between unaffected individuals and SCA patients, also showing that the SCA and SCT patients exhibited the class II skeletal pattern due to the mandibular setback. Moreover, maxillary expansion was not detected by traditional morphometrics, which is at odds with the geometric morphometrics findings. Even though both studies were performed on the same population, differing only in the methodology, geometric morphometrics findings indicate morphological differences among the groups.
With advances in multivariate statistics and computer technology in recent decades, a statistical tool known as geometric morphometrics, which is widely used for investigating the shape of organisms, has been developed. This technique allows for the detection of morphological changes associated with the variables of interest.13 Traditional morphometrics uses linear distances, indices or angles. Usually, these methods cannot preserve geometric data, which hinders the detection of changes in shape. It is often necessary to standardize the variables and there are some limitations regarding scale/size effects.22 Geometric morphometrics has proven to be a thriving method, with a great capacity for the identification of differences.20
Based on the assessed shapes, it is possible to state that the SCA patients differ from those in the other groups in all statistical estimations and that the major differences are observed in the external auditory meatus, at the base of the occipital bone, in the mandibular symphysis, and in the upper central incisor, showing a tendency towards buccal positioning of the upper central incisor and mandibular setback. The SCT group had a similar but statistically different pattern. Pithon et al.10 obtained similar findings for the maxillary prognathism and mandibular setback between the SCA and SCT patients, when compared to the control group. This finding is in line with the study conducted by Licciardello, Bertuna, Samperi,23 which assessed the craniofacial morphology of 36 patients with sickle cell disease and 36 volunteers without the disease, aged 18.5–51 years, in which the upper incisors were significantly more buccally positioned.
A study carried out by Maia et al.6 with 50 SCA patients in northern Minas Gerais, Brazil, showed a shorter mandibular length in 76% of the SCA patients. The tendency towards mandibular setback was also described by Altemus and Epps.24 Another study, conducted by Souza et al.,25 assessed the skeletal craniofacial features of 30 black individuals with SCA, aged 20–46 years, in Juiz de Fora, Minas Gerais, Brazil, and concluded that the major craniofacial bone changes were maxillary protrusion, mandibular setback, enlarged mandibular plane and convex facial profile, with a class II pattern. It was suggested that SCA might play a role in craniofacial skeletal defects.
Despite the small similarities in the shape of craniofacial structures between SCA and SCT patients, such as maxillary prognathism, with lower inclination of the upper central incisor and mandibular indentation, they still present statistically significant differences (p < 0.01). This is expected because, according to the Ministry of Health, 22 SCT is not a disease and is not a milder form of sickle cell disease or a latent form that could trigger the disease, depending on the circumstances. In addition, the life expectancy is similar to that observed in individuals without SCT, and the carrier status should not have any impact on lifestyle and quality of life.26
The identification and classification of SCA and SCT patients and unaffected individuals in several fields, including dentistry, are of paramount importance because, even though carriers of SCT do not require any special treatment, they must be informed that if they have children with another SCT carrier, they can conceive a child either with or without SCT or with SCA.2 In patients with sickle cell disease, treatment requires extra care and special attention to the risks for infections, including those associated with the techniques used by dental surgeons. In these patients, the prevention of infections is crucial and antibiotic prophylaxis is mandatory.27 Moreover, caution should be exercised when performing anesthetic and surgical procedures.28
Other studies conducted on individuals with other pathologies that involve craniofacial anomalies detected significant differences in the results of geometric morphometrics. Wellens et al.29 did not find any differences in the average shapes between men and women, but they observed allometric effects. In our study, there were neither differences in sex characteristics nor allometric effects, probably because most of the patients were older than 20 years (69%). In a study by Toro-Ibacache et al.30 aimed at assessing the morphofunctional consequences of the treatment of cleft palate on craniofacial growth, patients surgically treated for a unilateral cleft palate had larger variations in shape, when compared to those without a cleft palate, presenting mainly a retrognathic maxilla, a vertically elongated face, a more open mandibular angle and a more acute linear angle.
As inferred by Siğirli and Ercan,12 geometric morphometrics has a great potential for the investigation of the effects of environmental factors and of diseases in organs or organisms. This method allows for the identification of peculiarities in shape, the performance of a statistical analysis and the independent assessment of the variation in the shape of structures, based on landmarks.24
From the knowledge of the craniofacial format, it is easier to know the origin and location of morphological changes, which will contribute significantly to the orthodontic treatment and treatment choice, especially for patients with sickle cell anemia and sickle cell trait. As a limitation, it is associated with the cross-sectional study that often presents the limitation of the impossibility of indicating the causal reasons for the outcome.
ConclusionIndividuals with sickle cell disease show variations in the shape of craniofacial structures, especially in the external auditory meatus and at the base of the occipital bone. They also have a mandibular setback and a large upper central incisor inclination, showing a tendency towards protrusion. Geometric morphometrics is efficient in the categorization of SCA, SCT and unaffected groups, based on craniofacial features.
Conflicts of interestThe authors declare no conflicts of interest.