Coronavirus disease-2019 (COVID-19) caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) has caused global health crisis. Initially considered a respiratory tract pathogen, it can cause multiple organ dysfunction and present with a wide variety of complications (gastrointestinal, neurological, hematological, thromboembolic, immune and cardiovascular). Complications and outcomes may depend based on the severity and comorbidities of the infected patient. For optimal management of these patients, understanding of various systemic manifestations and complications of SARS-CoV2 is vital.
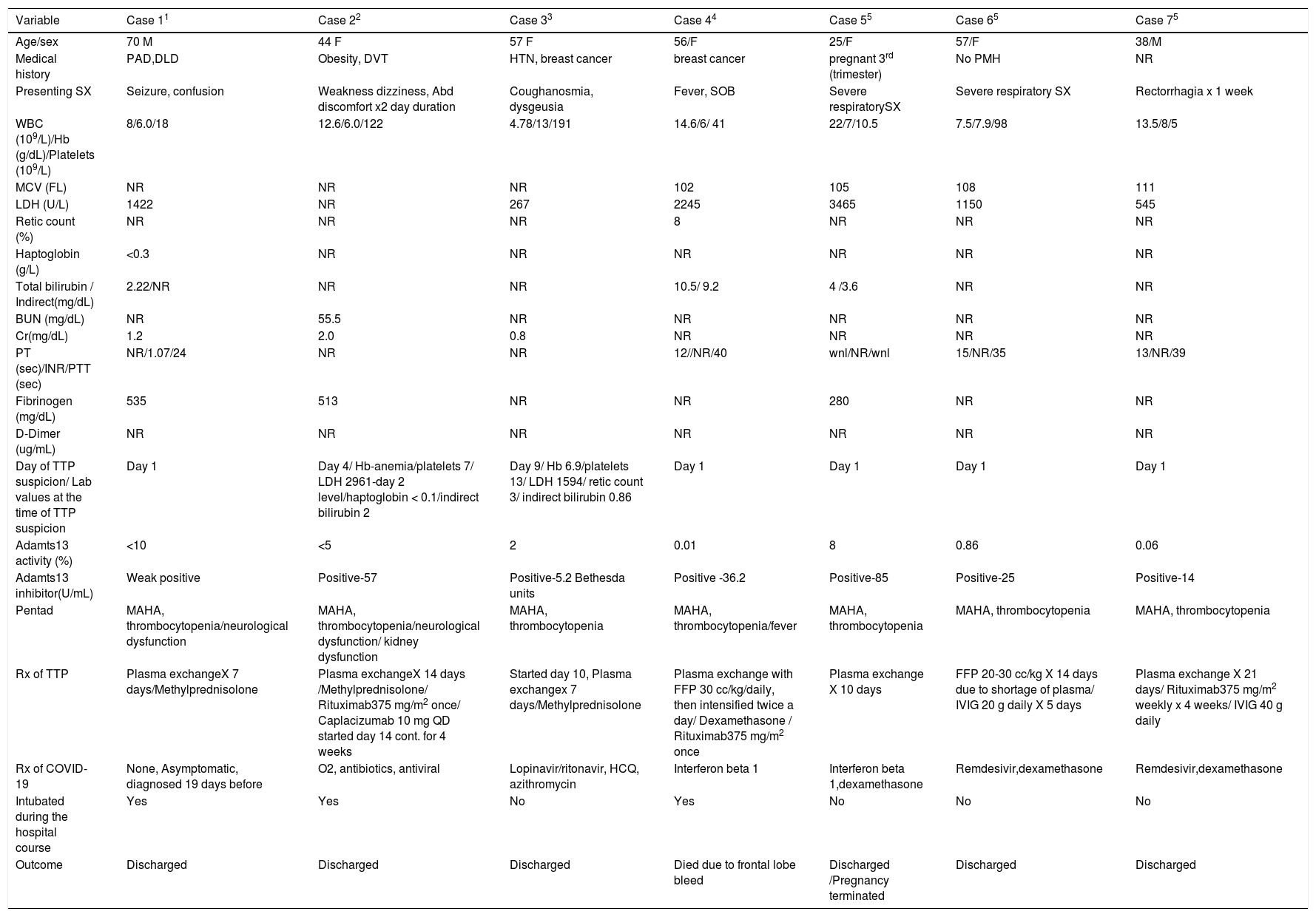
Limited literature is available regarding Thrombotic thrombocytopenic purpura (TTP) in COVID-19. We did an extensive literature review on COVID-19 associated TTP. We searched PubMed for this literature review using search terms ‘COVID-19 and TTP, ‘COVID-19 and Thrombotic thrombocytopenic purpura”. All the case reports with COVID-19 associated TTP so far were reviewed, and relevant data were abstracted from these studies. COVID-19 diagnosis was made by PCR assay except in one case by serological positive SARS-CoV-2 IgG positive (case 4). Table 1 summarizes the clinical characteristics, laboratory values, and outcome. 1-5
summarizes the clinical characteristics, laboratory values, and outcome.
| Variable | Case 11 | Case 22 | Case 33 | Case 44 | Case 55 | Case 65 | Case 75 |
|---|---|---|---|---|---|---|---|
| Age/sex | 70 M | 44 F | 57 F | 56/F | 25/F | 57/F | 38/M |
| Medical history | PAD,DLD | Obesity, DVT | HTN, breast cancer | breast cancer | pregnant 3rd (trimester) | No PMH | NR |
| Presenting SX | Seizure, confusion | Weakness dizziness, Abd discomfort x2 day duration | Coughanosmia, dysgeusia | Fever, SOB | Severe respiratorySX | Severe respiratory SX | Rectorrhagia x 1 week |
| WBC (109/L)/Hb (g/dL)/Platelets (109/L) | 8/6.0/18 | 12.6/6.0/122 | 4.78/13/191 | 14.6/6/ 41 | 22/7/10.5 | 7.5/7.9/98 | 13.5/8/5 |
| MCV (FL) | NR | NR | NR | 102 | 105 | 108 | 111 |
| LDH (U/L) | 1422 | NR | 267 | 2245 | 3465 | 1150 | 545 |
| Retic count (%) | NR | NR | NR | 8 | NR | NR | NR |
| Haptoglobin (g/L) | <0.3 | NR | NR | NR | NR | NR | NR |
| Total bilirubin / Indirect(mg/dL) | 2.22/NR | NR | NR | 10.5/ 9.2 | 4 /3.6 | NR | NR |
| BUN (mg/dL) | NR | 55.5 | NR | NR | NR | NR | NR |
| Cr(mg/dL) | 1.2 | 2.0 | 0.8 | NR | NR | NR | NR |
| PT (sec)/INR/PTT (sec) | NR/1.07/24 | NR | NR | 12//NR/40 | wnl/NR/wnl | 15/NR/35 | 13/NR/39 |
| Fibrinogen (mg/dL) | 535 | 513 | NR | NR | 280 | NR | NR |
| D-Dimer (ug/mL) | NR | NR | NR | NR | NR | NR | NR |
| Day of TTP suspicion/ Lab values at the time of TTP suspicion | Day 1 | Day 4/ Hb-anemia/platelets 7/ LDH 2961-day 2 level/haptoglobin < 0.1/indirect bilirubin 2 | Day 9/ Hb 6.9/platelets 13/ LDH 1594/ retic count 3/ indirect bilirubin 0.86 | Day 1 | Day 1 | Day 1 | Day 1 |
| Adamts13 activity (%) | <10 | <5 | 2 | 0.01 | 8 | 0.86 | 0.06 |
| Adamts13 inhibitor(U/mL) | Weak positive | Positive-57 | Positive-5.2 Bethesda units | Positive -36.2 | Positive-85 | Positive-25 | Positive-14 |
| Pentad | MAHA, thrombocytopenia/neurological dysfunction | MAHA, thrombocytopenia/neurological dysfunction/ kidney dysfunction | MAHA, thrombocytopenia | MAHA, thrombocytopenia/fever | MAHA, thrombocytopenia | MAHA, thrombocytopenia | MAHA, thrombocytopenia |
| Rx of TTP | Plasma exchangeX 7 days/Methylprednisolone | Plasma exchangeX 14 days /Methylprednisolone/ Rituximab375 mg/m2 once/ Caplacizumab 10 mg QD started day 14 cont. for 4 weeks | Started day 10, Plasma exchangex 7 days/Methylprednisolone | Plasma exchange with FFP 30 cc/kg/daily, then intensified twice a day/ Dexamethasone / Rituximab375 mg/m2 once | Plasma exchange X 10 days | FFP 20-30 cc/kg X 14 days due to shortage of plasma/ IVIG 20 g daily X 5 days | Plasma exchange X 21 days/ Rituximab375 mg/m2 weekly x 4 weeks/ IVIG 40 g daily |
| Rx of COVID-19 | None, Asymptomatic, diagnosed 19 days before | O2, antibiotics, antiviral | Lopinavir/ritonavir, HCQ, azithromycin | Interferon beta 1 | Interferon beta 1,dexamethasone | Remdesivir,dexamethasone | Remdesivir,dexamethasone |
| Intubated during the hospital course | Yes | Yes | No | Yes | No | No | No |
| Outcome | Discharged | Discharged | Discharged | Died due to frontal lobe bleed | Discharged /Pregnancy terminated | Discharged | Discharged |
We found a total of ten patients. 3 patients were excluded as ADAMTS 13 activity and inhibitor level was not reported.6-8 The median age of patients was 56 years (range 25–70 years). Out of 7 patients, 2 were male. Common comorbidities were – peripheral vascular disease, dyslipidemia, obesity, deep venous thrombosis, hypertension, breast cancer. Presenting symptoms were seizure, confusion, weakness, dizziness, abdominal pain, anosmia, dysgeusia, cough, fever, shortness of breath and rectorrhagia. 1 patient was pregnant at the time of TTP diagnosis. The complete workup of the 7 patients is outlined in Table 1. MAHA and thrombocytopenia were the most common presenting signs among the classical pentad. TTP can present concurrently with COVID-19 related respiratory symptoms or sequentially (case 1- presented 19 days later after COVID-19 infection). In case 1, the patient was hospitalized due to TTP and he had a history of positive RTPCR for SARS-COV-2 19 days prior to presentation. It is difficult to distinguish whether there is a cause-effect relationship between the diseases or TTP and COVID infection was incidental concomitant non-related events. Out of 7 patients, 5 were suspicious for TTP on day 1 of presentation. In 2 patients’ day of TTP suspicion was day 4 (case 2), and day 9 (case 3). In all the 7 patients ADAMTS 13 activity level was less than 10 % and ADAMTS 13 inhibitor was positive. The PLASMIC score could not be calculated in due to lack of the required variables in the reported cases. All the patients were treated with plasma exchange except 1 patient (case 6) who was treated with fresh frozen plasma. Only 1 patient (case 2) was treated with the Caplacizumab. Rituximab was used in 3 patients (case 2,4,7). Steroids (Methylprednisolone / dexamethasone) were used in the management of all the 7 patients – as such for TTP or part of the treatment for COVID-19. IVIG was used in 2 patients (case 6,7). Drugs used for the treatment of COVID-19 are outlined in Table 1. Out of 7 patients, 6 survived and 1 died (case 5).
Thrombotic thrombocytopenic purpura (TTP) is a rare medical emergency. The classical pentad of microangiopathic hemolytic anemia (MAHA), thrombocytopenia, neurological dysfunction, kidney dysfunction and fever are seen less than 10 percent of the patients.9 A high index of suspicion is required for a timely diagnosis. Early diagnosis is crucial as without treatment TTP is associated with high mortality rate. TTP is caused by decreased activity of the plasma metalloproteinase ADAMTS 13 (A Disintegrin And Metalloproteinase with a Thrombospondin type 1 motif, member 13), the key enzyme involved in the cleavage of ultra-large (von Willebrand Factor) VWF multimers into smaller less procoagulant multimers. TTP could be congenital due to mutations in the ADAMTS 13 gene or acquired due to the formation of autoantibodies against ADAMTS 13.
Viruses are known trigger factors in the pathogenesis of thrombotic microangiopathies.10 The exact pathological mechanism leading to the TTP in COVID-19 infected patients is not well understood at present, possibilities include direct endothelial injury, high inflammatory state associated with cytokine storm or increased procoagulant factors like factor VIII, von Willebrand factor, fibrinogen.11 Pascreau et al. analyzed 70 patients with COVID-19 pneumonia in regard to ADAMTS 13 antigen, plasma VWF activity and antigen and observed a marked increase in VWF levels and an associated moderate deficiency in ADAMTS 13.12 Direct viral infection of the endothelial cell leads to the release of VWF from endothelial storage sites. The ADAMTS13 is partially trapped to the endothelial surface where it cleaves the nascent unusually large VWF on the endothelial surface resulting in a mild to moderate decrease of circulating ADAMTS13. However, in this case series, no major thrombocytopenia or hemolytic anemia was noted. Thrombocytopenia (less than 150 × 109per L) was observed only 23% of patients at the admission with a median of 128 × 109 per L (ranging from 63 to 148 × 109/L). In our review antibody against ADAMTS13 was observed in all the cases likely suggesting multifactorial pathophysiology (ADAMTS13 inhibitors ADAMTS13‐von Willebrand factor axis disequilibrium) for the trigger of thrombotic microangiopathy in COVID-19 patients.
Hematological abnormalities such as anemia and/or thrombocytopenia can occur as such in COVID-19 patients. Thrombocytopenia is common in COVID-19 patients and can be more significant in severe cases. Possible mechanisms include a) aggregation and thrombosis in lungs resulting in platelet consumption, b) infection of bone marrow resulting in decreased production or c) triggering of an auto-immune response leading to increased destruction.13 Anemia in COVID-19 could be due to multiple etiologies; anemia related to inflammation or bleeding due to anticoagulation or autoimmune hemolytic anemia.14 Although direct causal association between COVID-19 and TTP is difficult, clinicians should have a high index of suspicion for TTP in the appropriate clinical scenario with relevant laboratory features.
Plasma exchange and immunosuppression are the mainstay of treatment for TTP. Caplacizumab a novel agent- inhibits the vWF-platelet glycoprotein-Ib interaction, blocks the adhesion of platelets to vWF multimers.15 Health care providers should be aware of this life threatening complication of COVID-19 so that prompt and appropriate interventions can be undertaken if it is suspected or confirmed.