During the COVID-19 pandemic, special attention has been addressed in cancer care to mitigate the impact on the patient’s prognosis. We addressed our preparation to face COVID-19 pandemic in a Hematological and Stem Cell Transplant Unit in Brazil during the first two months of COVID-19 pandemic and described COVID-19 cases in patients and health care workers (HCW). Modifications in daily routines included a separation of area and professionals, SARS-CoV-2 screening protocols, and others. A total of 47 patients and 54 HCW were tested for COVID-19, by PCR-SARS-CoV-2. We report 11 cases of COVID-19 in hematological patients (including 2 post stem cell transplant) and 28 cases in HCW. Hematological cases were most severe or moderate and presented with several poor risk factors. Among HCW, COVID-19 were mostly mild, and all recovered without hospitalization. A cluster was observed among HCW. Despite a decrease in the number of procedures, the Transplant Program performed 8 autologous and 4 allogeneic SCT during the period, and 49 onco-hematological patients were admitted to continuing their treatments. Although we observed a high frequency of COVID-19 among patients and HCW, showing that SARS-CoV-2 is disseminated in Brazil, hematological patients were safely treated during pandemic times.
Since all the world has been facing COVID-19 pandemic, concerns about hematological patients have been addressed.1,2 New diagnosis of acute or chronic leukemia, lymphoma, multiple myeloma is still being made, and patients must go on in their treatments to maintain response and prognosis. There are few reports about hematologic patients and COVID-19 until now, but they reported more severe disease in this population. 3–7 The rationale is that hematologic cancer confers immunosuppression by itself or by its treatment, and it is already known that the development of any severe infection can modify the treatment schedule, impacting prognosis. Another special issue in COVID-19 scenario is about the access and the quality care of cancer therapy since there are overcrowded hospitals and deficiencies in blood supplies.
COVID-19 was first noted in Brazil at the end of February, and a few days after, community transmission was documented. In the last two months, it spread to all Brazilian regions. Brazil faces severe difficulty in access diagnosis tests for all, so testing has been used mostly for individuals in risk group conditions, health care professionals, or patients who require hospitalizations.8
In this manuscript, we address our preparation to face the COVID-19 pandemic during the first two months and describe COVID-19 documented cases in patients and health care workers (HCW): from diagnosis to outcomes.
MethodsThis is a case series study from hematological patients treated from March 12th to May 21th in Complexo Hospitalar de Niterói, a quaternary level hospital, reference for stem cell transplantation (SCT), and solid organ transplant in Rio de Janeiro, Brazil. The study period coincides with the first 2 months after the documentation of community transmission of COVID-19 in Brazil. The hospital has a SCT Program since 2007 and performs a median of 100 autologous and 30 allogeneic SCT (related and unrelated) per year. The transplant unit (UTX) has 26 HEPA filtered single-room beds and receives patients for SCT and also hematological patients for chemotherapy and immunotherapy. A Day-Clinic hospital with 15 beds also integrates the unit. This study was approved by the institutional ethics committee (number 30907420.1.0000.5455).
Protocols and data collectionSince WHO declared COVID-19 a public health emergency of international concern in January 31th, the Transplant Unit planned modifications regarding daily routines, in addition to intense modifications in all hospital. After WHO declared COVID-19 a pandemic, a COVID-19 Committee was formed to coordinate all actions and specific areas for COVID-19 patients where delimited, intended not to permit crossover of COVID-19 and other patients. Regarding the Transplant Unit, hematology staff, transplant infectious diseases specialist, and transplant coordination have generated guidelines to best balance the risk of baseline malignancies with the risk of COVID-19 infection and mortality. International and National recommendations were references for our actions, as well as reports from other transplant centers that had previously faced COVID-19. Care modifications included inpatients and outpatients; transplant indication timing; blood cell supply; and intensive care unit. Firstly, we focused on patient and family education about the importance of social distancing, hand hygiene, and masking. Written material was distributed to all. Restriction in people circulation inside the UTX has been applied, and several modifications were implemented before and ongoing the pandemic weeks. We limited inpatients caregiver to one, and visitors are strictly limited.
UTX entrance has been confined to a single point, where all patients, families, and HCW are screened regarding symptoms and exposition. Patients with any symptoms regarding COVID-19 are masked and transferred to a specific Emergency Room to be tested. HCW or families that are symptomatic are not allowed to enter the Unit.
Since the beginning of the program, medical staffs’ surveillance of respiratory symptoms was intensified and testing all symptomatic has been routine.
Regarding patients care, all admissions of SCT, donor and recipient, scheduled chemotherapy, or unscheduled admissions such as febrile neutropenia, and others have been clinical and laboratory screened by SARS-CoV-2 PCR.
We gave continued training regarding secure, IPE, hospital and transplant flows, intubation, and others for all staff, and relevant data have been shared with all.
For this analysis, epidemiological, clinical, and laboratory data from COVID-19 cases were reviewed, and the outcome described. For HCW, we performed a web questionnaire. This questionnaire was applied twice, with 30 days apart, to identify COVID-19 cases.
Data were reported as frequencies, proportions, or medians. The chi-squared test was applied to compare frequencies and rates. Kaplan- Meir was applied to survival analyses, and curves were compared by log-rank. We defined a statistically significant p-value lower than 0.05.
ResultsSARS-CoV-2 testing and screeningA total of 47 onco-hematologic patients were tested for COVID-19 in the Unit. The screening was performed using SARS-CoV2 PCR for all cases. The test was applied to 30 symptomatic and 17 asymptomatic patients. Screened asymptomatic patients were: SCT donors (n = 4), SCT recipients (n = 10), hematological patients before scheduled chemotherapy (n = 3). No positive test was noted in asymptomatic screening, but only in symptomatic patients (10 cases; 33% of symptomatic; 21% of all tested). A COVID-19 case performed the test in other hospital, but she was transferred to our hospital to treatment.
Screening was done in 54 health care workers: 40 symptomatic and 14 asymptomatic justified by exposure to SARS-CoV-2. Only symptomatic HCW tested positive (28 cases; 70% of symptomatic, 52% of tested). Frequencies of COVID-19 in tested and symptomatic HCW were higher compared to hematological patients (52% vs. 21%; p = 0.003 and 70% vs. 33%; p = 0.005; respectively).
COVID-19 cases in hematological and transplant patientsEleven onco-hematological patients were diagnosed with COVID-19, including two recipients of haploidentical stem cell transplant (both late phase post SCT - after D + 100), and 9 patients with onco-hematological malignancies. Clinical and laboratory data are shown in Table 1.
Characteristics of hematological patients with COVID-19.
| Characteristics | n = 11 (%) |
|---|---|
| Age, median [range] (years) | 41 (3 – 83) |
| Male, n (%) | 6 (55) |
| Baseline disease, n (%) | |
| 3 (27) |
| 3 (27) |
| 1 (9) |
| 1 (9) |
| 1 (9) |
| 2 (18) |
| Previous SCT, n (%) | |
| 0 |
| 2 (18) |
| Treatment phase, n (%) | |
| 2 (18) |
| 4 (36) |
| 3 (27) |
| 2 (18) |
| COVID-19 symptoms, n (%) | |
| 9 (82) |
| 7 (64) |
| 5 (45) |
| 5 (45) |
| 2 (18) |
| 2 (18) |
| 1 (9) |
| 1 (9) |
| COVID-19 staging, n (%) | |
| 2 (18) |
| 5 (45) |
| 4 (36) |
| Comorbidities, n (%) | |
| 3 (27) |
| 2 (18) |
| 3 (27) |
| 1 (9) |
| 1 (9) |
| 0 |
| 4 (36) |
| Laboratorial findings during COVID-19 | |
| 803 (<100 – 13,691) |
| 5 (45%) |
| 503 (<100 – 2312) |
| 8 (73) |
| 5 (45) |
| 18.62 (0.14 – 69) |
| 2.56 (0.59 – 7.9) |
| 2 |
| 29 (14 – 38) |
| 5 |
| 630 (527 – 943) |
| 46 (< 5 – 1099) |
| 1,215 (189 – 4957) |
| Lung CT scan, n = 9 (%) | |
| 2 (22) |
| 4 (44) |
| 3 (33) |
| Complications, n (%) | |
| 10 (83) |
| 6 (55) |
| 4 (36) |
| 5 (45) |
| 2 (18) |
| 1 (9) |
| Outcome, n (%) | |
| 5 (45) |
| 1 (10) |
| 5 (45) |
*01 patient with severe disease had chronic renal disease in hemodialysis.
SCT: Stem cell transplantation; CT: Computed tomography.
Regarding haploidentical patients, they were treating chronic severe GVHD at the moment of COVID-19, and immunosuppression included corticosteroids. Regarding onco-hematological patients, two episodes of COVID-19 were noted in untreated newly diagnosed patients. Most cases were moderate or severe (n = 9; 82%), and 5 (45%) died due to COVID-19. One patient is still on treatment, but recovering.
COVID-19 cases in HCW from transplant unitData from 84 HCW were collected, including 37 physicians and 18 registered nurses (RN). It represents 100% of physicians and RN who works in the SCT Unit, as routine or consulting physicians. Forty-six (55%) HCW have employment in more than one hospital, and 37 (44%) work at least 4 days per week in the UTX. Fifty-four HCW performed at least one test during the study. COVID-19 were documented in 28 (33%) of HCW: 21.6% of physicians and 44.4% of RN. All HCW were symptomatic, but the disease intensity was mild or moderate. No hospitalization was required, and all HCW recovered. Frequency of COVID-19 in HCW who works in another hospital (46% vs. 60%; p = 0.21) or works at least 4 days per week (50% vs. 39%; p = 0.36) were not different from those who work only in our hospital or few days per week in the UTX.
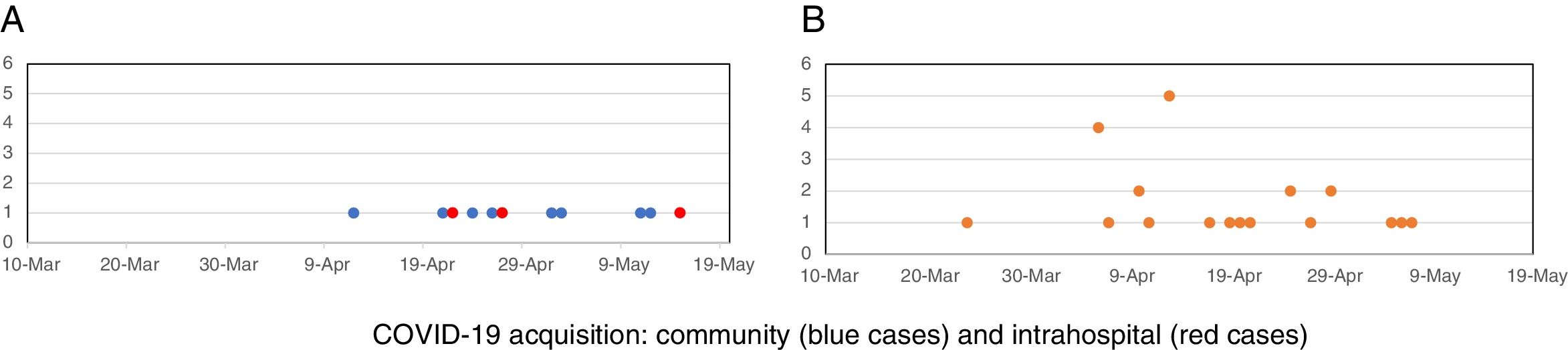
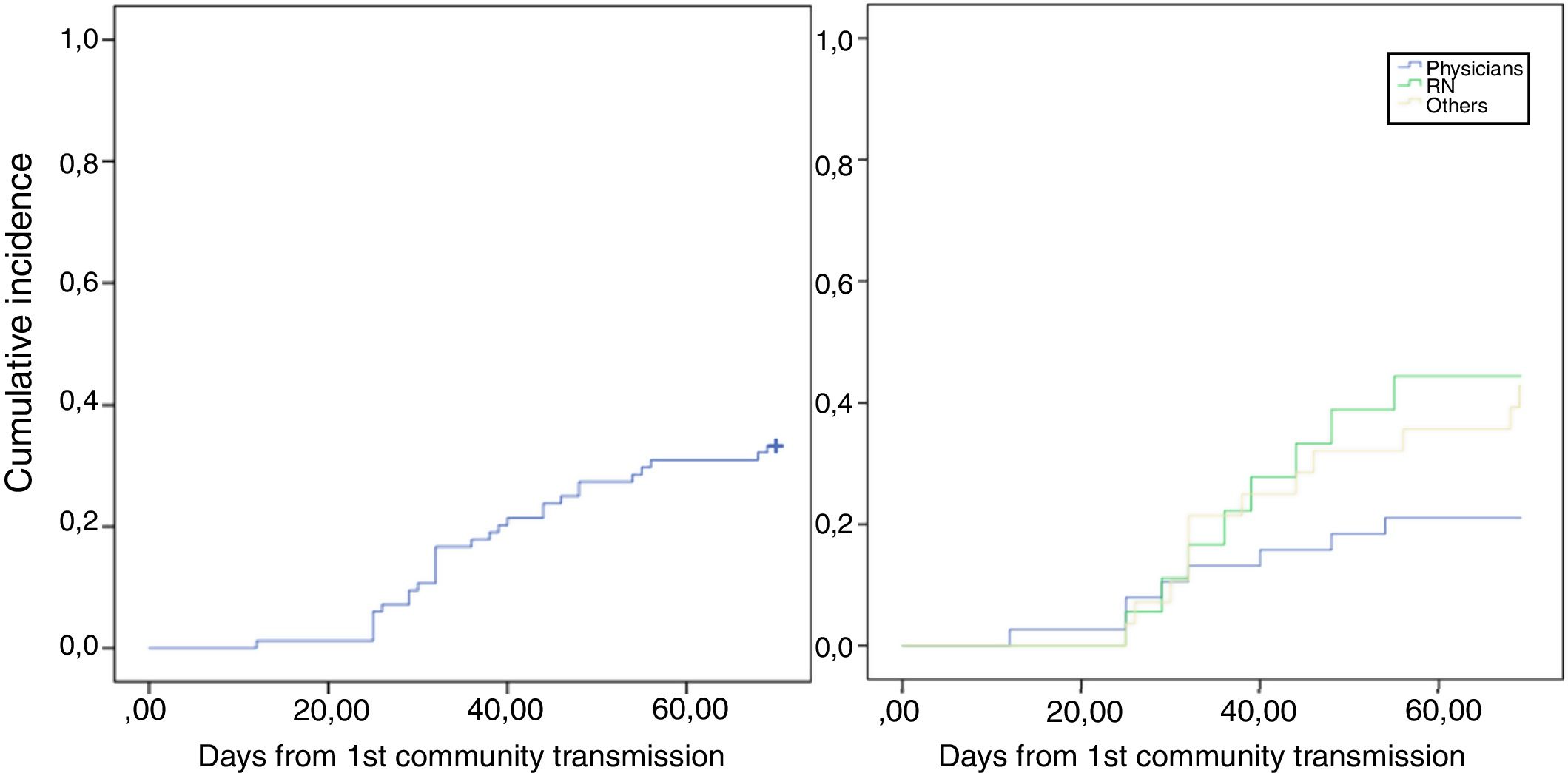
Timeline of COVID-19 in onco-hematological patients and HCWThe timeline of COVID-19 cases is represented in Fig. 1A. Our first case of COVID-19 in hematological patients was documented on March 30th, in another hospital, and the number of cases increased after April 15th. Three patients developed COVID during hospitalization, but in two, a close relative was the probable source of infection. In the other patient, the source could be intrahospital. In all the others, COVID-19 symptoms were present at hospitalization, and the patient was treated outside UTX. Regarding HCW, COVID-19 cases started earlier comparing to hematological patients, but most HCW cases occurred after the second week of April, with a cluster distribution of thirteen cases in 2 weeks. (Fig. 1B) After this cluster, training and screening were intensified, and universal use of masks during all hospital stay, including HCW common areas, was then implemented. HCW cases still occurred but in more regular distribution during weeks. Fig. 2.1 shows the cumulative incidence of COVID-19 in HCW, and Fig. 2.2, the incidence by professional categories (p = 0.13).
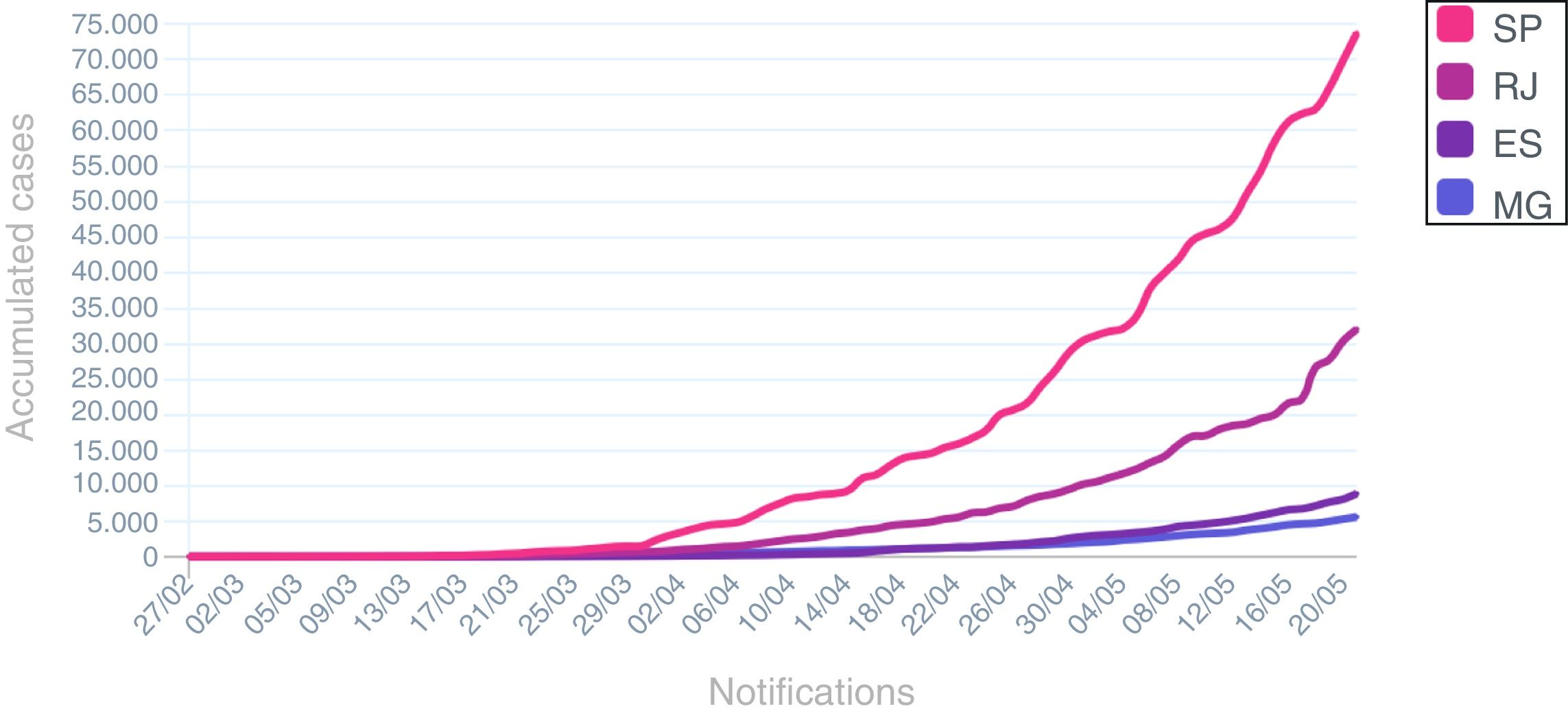
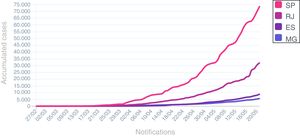
The spread in number of COVID-19 in hematological patients and in HCW after second week of April is similar to cases distribution in Rio de Janeiro State during the same period (Fig. 3).
Cumulative cases of COVID-19 in south-eastern States in Brazil during pandemic. SP: São Paulo State; RJ: Rio de Janeiro State; ES: Espírito Santo State; MG: Minas Gerais State.
During the same two months, a total of 61 hematological patients were admitted in the UTX. Autologous and allogeneic SCT was performed in eight and four patients, respectively. Despite two autologous patients (admission in UTX occurred before the documentation of community transmission in Brazil), all recipients and donors were screened by SARS-CoV-2 PCR before admission or stem cell harvest. No COVID-19 was observed in SCT during the early phase.
DiscussionOur experience during the initial months of COVID-19 pandemic highlighted some observations: COVID-19 presents as moderate or severe cases in onco-hematological patients, and the mortality was high. High intensively treated patients or those with comorbidities were at worse risk for severe COVID. A large proportion of HCW experienced COVID-19, independently of the category of professionals. We could observe a cluster of COVID-19 in HCW, suggesting possible transmission within HCW. After intensified measures, the spread of cases in patients and HCW were similar to those reported in our State, and the Unit was maintained as a “COVID-19 free zone”. Regardless of the pandemic, the transplant program was continued but had a 50% reduction in autologous transplants procedures. Regarding allogeneic, we did not experience a reduction in transplants procedures. This workforce priories the importance of keep cancer treatment going, and so mitigate the impact of postponing SCT in high risk cancer patients.
The knowledge of COVID-19 is in progression. The disease was first documented a few months ago but is now spreading worldwide. Description of manifestations and outcomes from specific sub-settings of patients is a mandatory issue for a better understanding of this new pathology and provides tools to face it. 10–13 Few reports addressed hematological patients. Data from China and France reported concerning outcomes: 85% and 40% mortality rates among hematological patients, respectively. 3,4 Most patients had other comorbidities despite hematological cancer, and the COVID-19 manifestation was frequently severe, requiring mechanical ventilation and intensive care in a high proportion of cases from both series. Data from Italy, including solid tumor patients, showed better outcomes than China and France series, but all authors concluded that COVID-19 has a worse evolution in cancer patients compared to non-cancer population. 5,6 In our series, the mortality rate was similar to France reports. We also find poor laboratory markers and severe pulmonary involvement at the onset of severe diseases.
On the other hand, mild and moderate cases at onset had uncomplicated evolution. It is important to address that their and our cases were all symptomatic patients that had a documented COVID-19. It is a crucial bias since asymptomatic or oligosymptomatic cases are not documented unless they require medical assistance or hospitalization for other reasons. There is a possible overestimation of complications and mortality rates.
Regarding HCW, we report a high incidence of COVID-19 regardless of the professional category, and we were unable to establish an association with work characteristics such as frequency and work in more than one hospital. HCW cases increased concomitantly with cases in our State, but we could observe a cluster at the beginning of transmission. This fact suggests that a possible transmission within HCW happened, as all COVID-19 onco-hematological patients were managed outside the UTX, and no case in the hospitalized patient was identified in the same period. The COVID-19 rate in HCW was higher than some reports from Italy, 5,14 but similar to first reports from China. 15 Cluster distribution was also noted in Singapore and China. 16,17 HCW is considered a high-risk group for infection and transmission, but fortunately most cases were mild. 18
This high incidence of COVID-19 in HCW highlighted the importance of intense screening, regular training, and adequate personal protective equipment to limit the spread and to reduce the risk for HCW to become infected. Any symptoms or suspected community exposition should be managed as a possible case, and the HCW must be removed from work until appropriate documentation. The frequency of testing positive was 70% in symptomatic HCW, a very high proportion compared to symptomatic onco-hematologic patients. Although, we did not find COVID-19 documentation in asymptomatic HCW, pre-symptomatic transmission is of great concern. Impact of asymptomatic HCW screening is not clear, but measures to reduce pre-symptomatic transmission must be implemented.16
Despite the pandemic, transplant programs from other countries maintained the program active, as postponing transplantation is not feasible in some scenarios. The European Society for Blood Marrow Transplantation (EBMT) and The Brazilian Society for Blood Marrow Transplantation (SBTMO) recommendations allow procedures if the patients' safety is maintained. 19,20 We decided to maintain the program active but limited the procedure to those in which the prognosis would be impacted by postponing transplantation. This decision was based on an intense collaboration of the institution as a whole and revised with daily results.10,21
Asia, Europe, and North America are in a different pandemic phase compared to Brazil. These regions are reopening, but Brazil has not achieved the peak of incidence yet and has an increasing number of infected and deaths daily. Unfortunately, all the world is exposed to second or more waves of COVID-19, until an efficient vaccine is available. SARS-CoV-2 will remain a concern for a long time. We hope our experience may contribute to a better comprehension of the disease in the onco-hematological scenario and improve the capacity to mitigate the negative impact in cancer care assistance during the Pandemic.
Conflict of InterestThe author declares no conflicts of interest.