Consensus of the Brazilian association of hematology, hemotherapy and cellular therapy on patient blood management
More infoPatient Blood Management (PBM) is a multidimensional approach that seeks to optimize the use of blood and its components in patients. This matter emerged as a response to the need to reduce unnecessary exposure to blood transfusions and their potential risks. In the past, blood transfusion was often overused resulting in complications and high costs. The advent of Patient Blood Management has caused a paradigm shift, highlighting anemia prevention, bleeding control and maximizing the production of blood cells by the organism itself. Patient Blood Management guidelines include the early identification of anemia, strategies to minimize blood loss during surgery, intraoperative blood conservation techniques, preoperative hemoglobin optimization and evidence-based approaches to the rational use of blood transfusions. Aiming to improve clinical outcomes, decrease transfusion-related complications and reduce associated costs, this multidisciplinary approach counts on doctors, nurses, pharmacists and other healthcare professionals. Based on research and clinical evidence, Patient Blood Management continues to evolve thereby promoting safer, more effective patient-centered practices. Its implementation has proven beneficial in various medical contexts thereby contributing to improvements in the quality of care provided to patients. Our goal with this Consensus is to present readers with a broad and diverse view of Patient Blood Management so that they have the building blocks to implement this new technique.
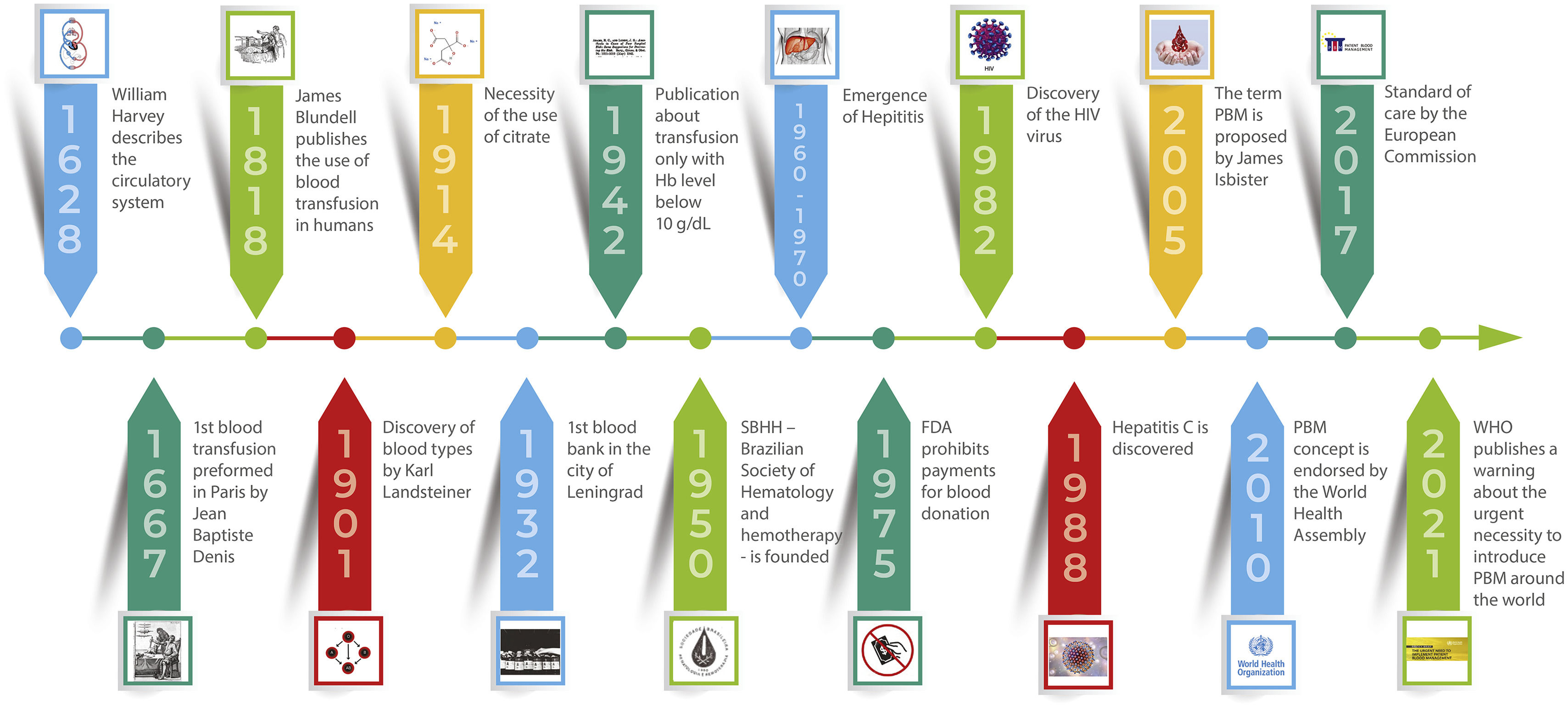
Blood has been used as a treatment for various diseases since ancient times. Pharaohs used blood to cure filariasis, while in ancient Rome, the blood of defeated gladiators was drunk to treat epilepsy. However, blood transfusion began to be considered in a more physiological way only after the description of the circulatory system by Harvey in 1628.1
The first blood transfusion in a human being was performed in Paris by Jean Baptiste Denis in 1667, who transfused blood from an animal to a young man.2 James Blundell, a London obstetrician, published the use of blood transfusions between humans in 1818, but frequent incompatibility reactions and the impossibility of storage were obstacles to the dissemination of the practice.3
The discovery of blood types in 1901 by the physician Karl Landsteiner reduced incompatibility reactions and the anticoagulation of blood using citrate, which allowed blood to be stored, was reported in 1914. In 1932, the first blood bank was created in the city of Leningrad. A recommendation based merely on expert opinion published in 1942 became the reference for decades; it was to transfuse when the patient had a hemoglobin (Hb) level below 10 g/dL.1
In response to the demand created by open heart surgery and advances in the treatment of trauma, the 1950s were marked by an intense increase in the use of blood. In 1950, the Brazilian Society of Hematology and Hemotherapy was founded, consolidating two sister specialties.4
Blood became a valuable commodity to be bought and sold. However, in 1975, with the emergence of post-transfusion hepatitis, which occurred mainly with blood obtained from donors who received payment, the Food and Drug Administration (FDA) prohibited this practice. Despite this, cases of non-A/non-B hepatitis still occurred frequently, and in 1982, the blood-borne HIV virus was discovered. In 1988, the hepatitis C virus was identified as the causative agent of non-A/non-B hepatitis. Safety measures were implemented, including donor screening and more effective testing to promote safer blood transfusions.1,5
Introduction to patient blood management – global and Brazilian scenariosCurrently, blood transfusion is one of the most commonly performed procedures during hospital admissions in the United States.6 In 2019, Brazil carried out more than 2.95 million transfusions,7 while the World Health Organization (WHO) estimates that more than 112 million bags of blood are collected annually around the world.8
On the other hand, a concept, which has been gaining momentum in the scientific world, is that of Evidence-Based Medicine (EBM), an approach to optimize medical decision-making, emphasizing the use of evidence from well-conducted research that proves the efficacy and safety of treatments. This term was first used by researchers at McMaster University, Canada, in the 1990s with the aim of incorporating clinical research findings into the decision-making process to bring more science to medicine.9 Thus, the EBM movement began to take shape only after blood transfusion had become a very widespread treatment.
In the conception of EBM, randomized clinical trials are considered the study type that provide the greatest scientific evidence. Although there are no randomized clinical trials comparing blood transfusion with placebo, 10,11 randomized clinical trials comparing a liberal transfusion strategy, where more is transfused to maintain higher Hb values, versus a restrictive one, where less is transfused to maintain lower Hb values, have been conducted. The Transfusion Requirements in Critical Care (TRICC) trial was the first to make this type of comparison at the end of the 90s. 12 This study, carried out on patients admitted to the Intensive Care Unit (ICU), concluded that there were no statistical differences in overall survival, but observed lower mortality in the restrictive group during hospitalization.
After the publication of the TRICC trial, other randomized clinical trials were carried out comparing restrictive transfusion strategies with liberal ones in different clinical situations, such as in cardiac surgery and digestive tract bleeding. These studies concluded that more restrictive transfusion strategies presented lower mortality and fewer adverse clinical outcomes. A systematic review with meta-analysis of randomized clinical trials confirmed these findings.13
Based on systematic reviews of randomized clinical trials, current guidelines recommend restrictive rather than liberal transfusion for most patients.14 However, these guidelines do not consider an important limitation of the clinical trials: the lack of a placebo control group. Trentino et al.15 mentioned that these studies were not designed to specifically test the effectiveness of transfusion as they do not compare blood transfusion with placebo.
Although there are no randomized clinical trials that have compared blood transfusion versus no transfusion, there is observational research that made this type of comparison. A systematic review with meta-analysis of these studies evaluated clinical outcomes in 272,596 critically ill patients and observed that in 42 of the 45 studies the risks of transfusions outweighed the benefits. In 17 of 18 studies, transfusion was an independent factor associated with higher mortality.16 An analysis of the Bradford Hill causality criteria concluded that all criteria were met regarding transfusion-associated adverse outcomes.17,18
The clinical effects observed with blood transfusion are mainly attributed to a phenomenon called transfusion-associated immunomodulation,19 the deleterious effects that the transfusion can cause on the recipient's immune system, and to the various changes that occur in red blood cells stored in blood bags. Denominated storage injury, these alterations, some of which are irreversible, include biomechanical, biophysical and morphological changes. 20 They start to appear after just a few hours of storage,21 thereby impairing the functioning of red blood cells and causing damage to the recipient's body.22,23
The emergence of patient blood managementGiven the scenario reported in the literature by Shander et al.,25 in which a significant portion of blood transfusions are considered inappropriate,24 and the accumulation of evidence that indicates that greater restriction of transfusions promotes better clinical outcomes,25 a new treatment approach called Patient Blood Management (PBM) is being recommended in the medical literature. 26 A systematic review with meta-analysis involving 235,779 patients concluded that the use of PBM is associated with a reduction in blood transfusions, as well as complications such as acute renal failure, infection, thromboembolic events, mortality and length of hospital stay. 27 In addition to promoting better clinical outcomes, scientific studies have concluded that PBM provides significant savings in financial resources.28,29
The term PBM was originally proposed in 2005 by James Isbister at a board meeting of the Medical Society for Blood Management and first appeared in the literature in 2008.30 The concept of PBM was endorsed in 2010 by the World Health Assembly through resolution WHA 63.12. In 2017, it was recommended as standard of care by the European Commission and, in 2019, by the American Society of Hematology.31 In 2021, the WHO published a warning about the urgent need to adopt PBM all around the world.32 A future article will address the definition and concept of this new medical approach.
ConclusionPBM represents an innovative and comprehensive approach to optimizing the use of blood and its components while prioritizing patient safety and well-being. By adopting evidence-based strategies for anemia prevention, bleeding control and the rational use of blood transfusions, this multidisciplinary approach has demonstrated significant benefits in improving clinical outcomes, and reducing complications and associated costs. With the continuous advancement of research and growing awareness of the importance of PBM, it is expected that its implementation will become increasingly widespread, providing quality, patient-centered care, contributing to improvements in the medical practice as a whole (Fig. 1).
Complementary virtual references- 1.
- PBM – Patient Blood Management: II Série de Webinar de Hemoterapia – link de acesso: https://youtu.be/PByJcH9gFzo
- 2.
- HEMO PLAY Podcast #Ep48 - Conheça o projeto PBM da ABHH – link de acesso: https://youtu.be/TyegH31× 7cI
- 3.
- WEBINAR COLABORATIVO SOBRE PATIENT BLOOD MANAGEMENT – link de acesso: https://youtu.be/V1-iIHN_KZo