Introduction: Flumatinib, a highly selective ABL kinase inhibitor, exhibits stronger inhibition of intracellular BCR-ABL tyrosine kinase activity, compared to Imatinib. However, there is limited research comparing the real-world efficacy and safety of flumatinib and dasatinib in patients with Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL). Objective: Investigating the differences in therapeutic efficacy and safety between flumatinib and dasatinib in combination with multi-drug chemotherapy for the treatment of newly diagnosed Ph+ ALL. Method: In this study, we assessed 43 patients with newly diagnosed Ph+ ALL (20 in the flumatinib group, 23 in the dasatinib group). Results: There were no significant differences in gender, age, fusion gene type, initial blood routine, bone marrow blast cell ratio or chromosome karyotype between the two groups. Within 1 month, there were no significant differences in the complete response (CR), major molecular response (MMR) or minimal residual disease (MRD) negativity rate between the flumatinib and dasatinib groups. Similarly, within 3 months, there were no significant differences in CR or MMR rates between the two groups. However, the rates of complete molecular response (CMR) and MRD negativity within 3 months were significantly higher in the flumatinib group, compared to the dasatinib group (P < 0.05). Additionally, the flumatinib group exhibited fewer adverse reactions compared to the dasatinib group. Conclusion: These findings suggest that flumatinib is a safe and effective tyrosine kinase inhibitor (TKI) for achieving CMR and MRD negativity in patients with Ph+ ALL, as supported by this small series of patients.
Acute lymphoblastic leukemia (ALL) is a malignant proliferative disease primarily originating from T or B lymphoid precursor cells. Among adult patients, ALL comprises approximately 20% of all acute leukemias. The Philadelphia chromosome (Ph) results from a translocation between chromosomes 9 and 22, leading to the formation of the BCR-ABL fusion gene.1 At the time of diagnosis, about 20% to 40% of adult patients with ALL exhibit the Philadelphia chromosome-positive (Ph+) status, characterized by the presence of BCR-ABL fusion gene abnormalities.2,3 The prognoses of Ph+ ALL patients have significantly improved with the incorporation of tyrosine kinase inhibitors (TKIs) in the chemotherapy regimen. Imatinib, the first-generation TKI, has significantly improved the efficacy and prognosis of Ph+ ALL patients. The introduction of second-generation TKIs, including nilotinib, dasatinib, and bosutinib, has further enhanced the response rates in Ph+ ALL patients. These advancements have not only improved the response rates, but have also extended the overall survival time for Ph+ ALL patients.4–6 Currently, TKIs combined with standard chemotherapy achieve a high complete response (CR) rate of 91% to 98% in Ph+ ALL. This approach also improves the 5-year overall survival (OS) rate to 40% to 70%.2,7 Flumatinib-mesylate is a second-generation TKI developed in China. There are few reports comparing the real-world efficacy and safety of flumatinib-based and dasatinib-based therapy in Ph+ ALL patients. This study aimed to analyze clinical data of newly diagnosed Ph+ ALL patients treated with flumatinib or dasatinib, combined with multi-agent chemotherapy. The primary objectives were to evaluate the efficacy and safety of flumatinib and dasatinib in the treatment of Ph+ ALL patients.
MethodsStudy design and participantsThis is a single-center real-world retrospective case-control study performed on 43 patients. Eligible patients were newly diagnosed Ph+ ALL. The key exclusion criteria included patients who had died before completing a course of treatment and patients who did not receive flumatinib or dasatinib.
The study protocol was reviewed and approved by the institutional review board or an independent ethics committee at all participating centers. The patient consent was waived due to the use of human material or data with identifiable information to conduct the research and, in this manner, the subjects can no longer be identified and the research project does not involve personal privacy or commercial interests.
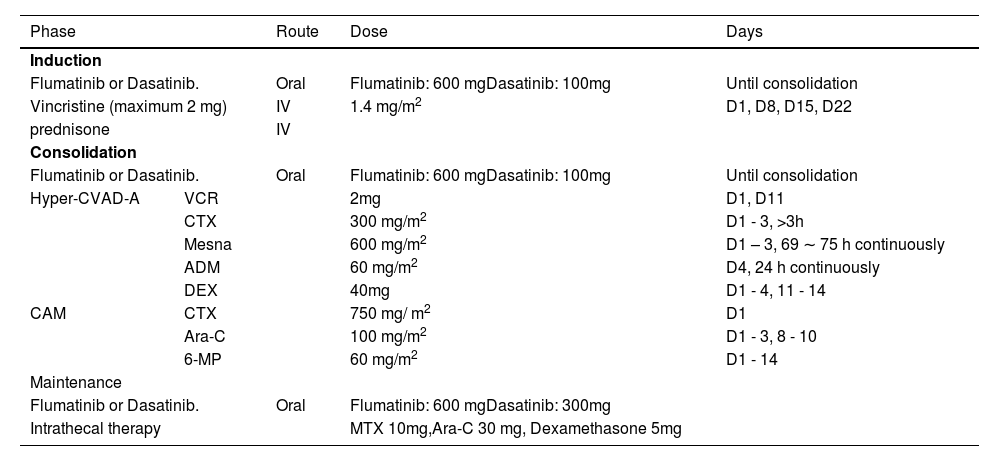
Chemotherapy regimenThe VP (vincristine + prednisone) regimen was used for induction chemotherapy. During the intensive consolidation treatment stage, hyper-CVAD-A (cyclophosphamide + doxorubicin + vincristine + dexamethasone + sodium 2-mercaptoethane sulfonate (mesna)) or CAM (cyclophosphamide + cytarabine + 6-mercaptopurine) regimens were administered according to the patient's condition and the related adverse reactions were treated. Some patients underwent sequential hematopoietic stem cell transplantation (HSCT) after CR. The triple intrathecal injection of methotrexate, dexamethasone and cytarabine was used to prevent or treat central nervous system leukemia during the treatment. ALL patients, once diagnosed as PH+ ALL, received TKIs (Table 1).
Chemotherapy schedule.
The follow-up was based on the medical record system and telephone follow-up and the follow-up deadline was set on April 30, 2023. Complete remission (CR) was defined as absolute neutrophil count ≥ 1.0 × 109/L, platelet count ≥ 100 × 109/L, no blast cells in peripheral blood and bone marrow blasts < 5%. The major molecular response (MMR) was defined as quantitative BCR-ABL copy number ≤ 0.1%. Complete molecular response (CMR) was defined as BCR-ABL copy number negative after CR. Relapse was defined as the presence of leukemic blasts in the bone marrow or the presence of extramedullary infiltration after remission. Overall survival (OS) was defined from the time of diagnosis until the end of follow-up or death. Progression-free survival (PFS) was defined as the time from the date of the first CR to the date of relapse or death due to other factors or the end of the follow-up and patients who were lost to the follow-up were followed until the date they were last known to be alive. Minimal residual disease (MRD) levels in bone marrow were detected using multi-parameter flow cytometry and the proportion of leukemia cells in the total number of nucleated cells in the bone marrow was used as the MRD value. An MRD ≥ 0.01% was considered positive, while an MRD < 0.01% was considered negative. Second-generation gene sequencing was employed to measure the levels of the BCR-ABL gene in bone marrow samples.
Adverse reactionsAll adverse events were processed according to the National Cancer Institute Common Adverse Event Terminology Criteria (NCI CAETC), version 5.0, being reported and graded. The observed adverse reactions included hematological and non-hematological reactions.
Statistical analysisThe SPSS 26.0 software was used for statistical analysis. The Fisher's exact probability method was used for numeric variables and the Mann-Whitney U test was used for categorical variables. The Kaplan-Meier method was used to calculate the overall survival (OS) and the progression-free survival (PFS). The log-rank test was used to compare the survival analysis between the two groups. The statistical test was two-sided and p < 0.05 was considered statistically significant.
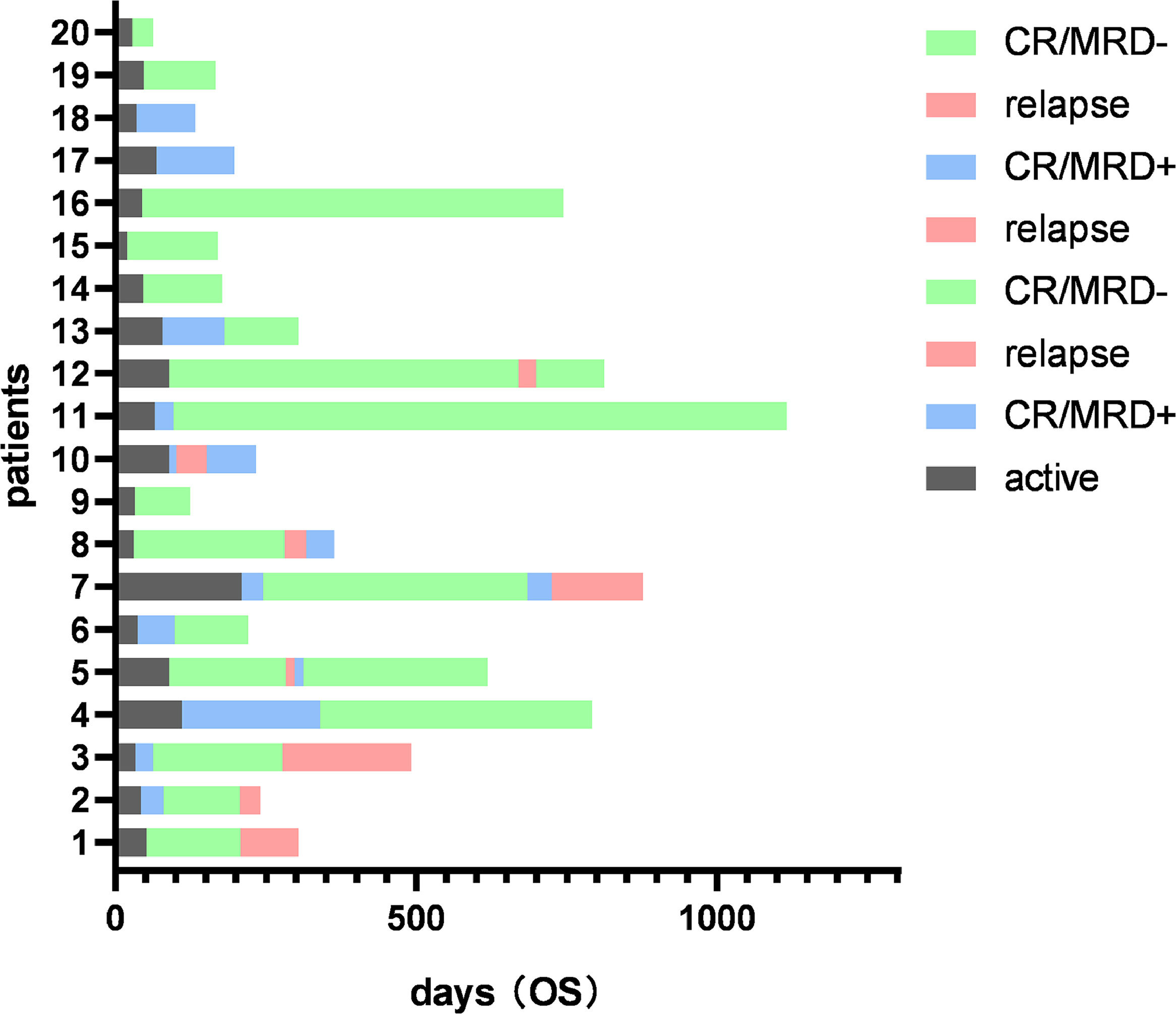
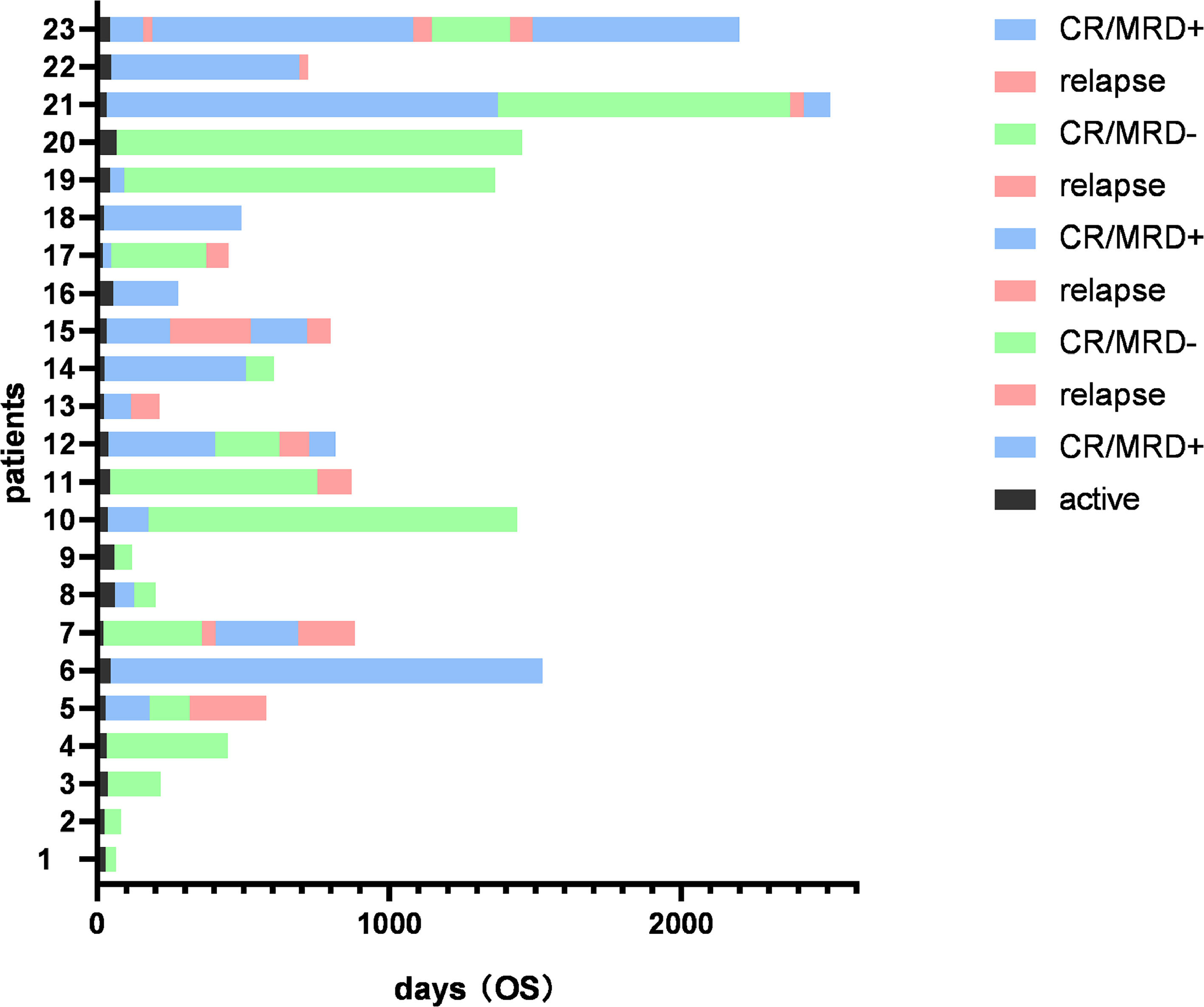
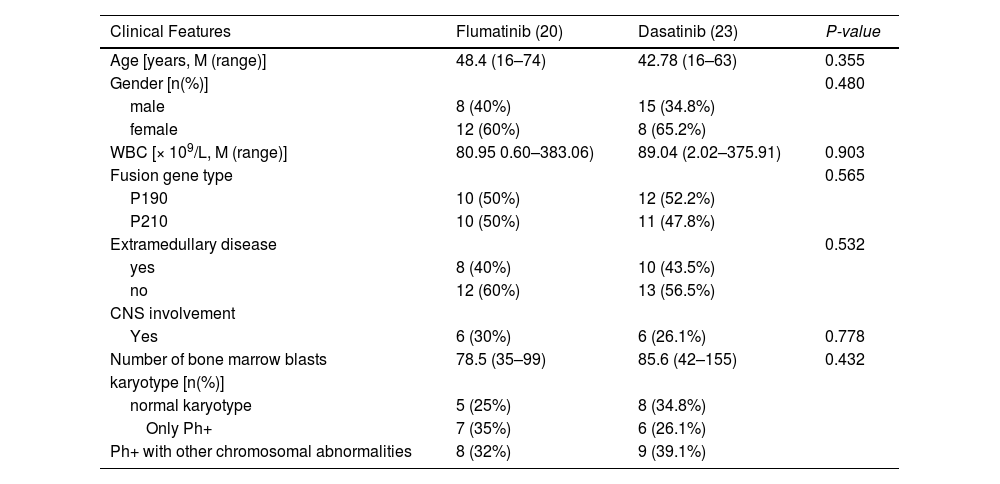
ResultsClinical characteristics of Ph+ all patientsFrom May 2018 to April 2023, forty-three patients were consecutively enrolled and, according to the different TKI uses, they were divided into the flumatinib group (n = 20) and the dasatinib group (n = 23). The median duration of the follow-up was 415 days (range, 83 – 2511 days). There was no significant difference in gender, age, fusion gene type, initial blood routine, bone marrow blast cell ratio, chromosome karyotype or the central nervous system (CNS) involvement at the disease presentation between the flumatinib group and the dasatinib group. Seven patients underwent HSCT after CR (four patients in the flumatinib group and three patients in the dasatinib group). Detailed clinical features are shown in Table 2 and the clinical course of the patients is shown in Figures 1 and 2.
Clinical characteristics of Ph+ acute lymphoblastic leukemia patients receiving flumatinib or dasatinib as first-line therapy.
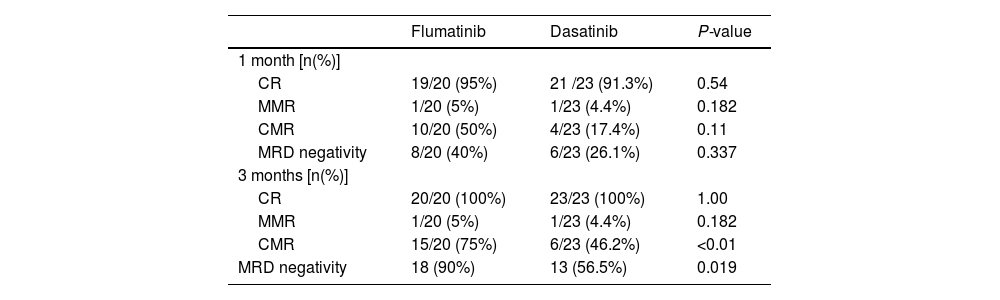
The flumatinib group had a CR rate, MMR rate and MRD negativity within 1 month of 95%, 5% and 50%, respectively. Within 3 months, the CR and MMR rate were 100% and 5%, respectively. For the dasatinib group, the CR rate, MMR rate and MRD negativity rate within 1 month were 91.3%, 4.4% and 26.1%, respectively. Within 3 months, the CR and MMR rates were 100% and 4.4%, respectively. There was no statistical significance in the aforementioned outcomes between the two groups. However, the flumatinib group showed superior results with a 3-month CMR rate and MRD negativity rate of 75% and 90%, respectively, compared to the 46.2% and 56.5%, respectively, of the dasatinib group. There was a statistically significant difference between the two groups (Table 3).
Clinical efficacy of flumatinib versus dasatinib in the treatment of patients with Ph+ acute lymphoblastic leukemia.
Note: CR: complete remission; MMR: major molecular response; CMR: complete molecular response; MRD: minimal residual disease; OS: overall survival; PFS: progression-free survival.
During the follow-up period, 8 patients (40%) in the flumatinib group and 11 patients (47.8%) in the dasatinib group experienced relapse, with no observable statistically significant difference. Screening for ABL mutations was conducted in all patients with relapse. Among the relapsed patients in the flumatinib group, four patients were found to have T315I mutations and were subsequently treated with olverembatinib. Presently, two out of the four relapsed patients in this group have achieved disease-free status, one patient died due to relapse and one patient died due to sepsis. In the dasatinib group, a total of eleven patients experienced relapse, of whom three patients (including one with the T315I mutation) were switched to a flumatinib-based therapy. Currently, one patient in this group is disease-free and two patients have died due to relapse. The remaining relapsed patients either discontinued treatment or were lost to the follow-up.
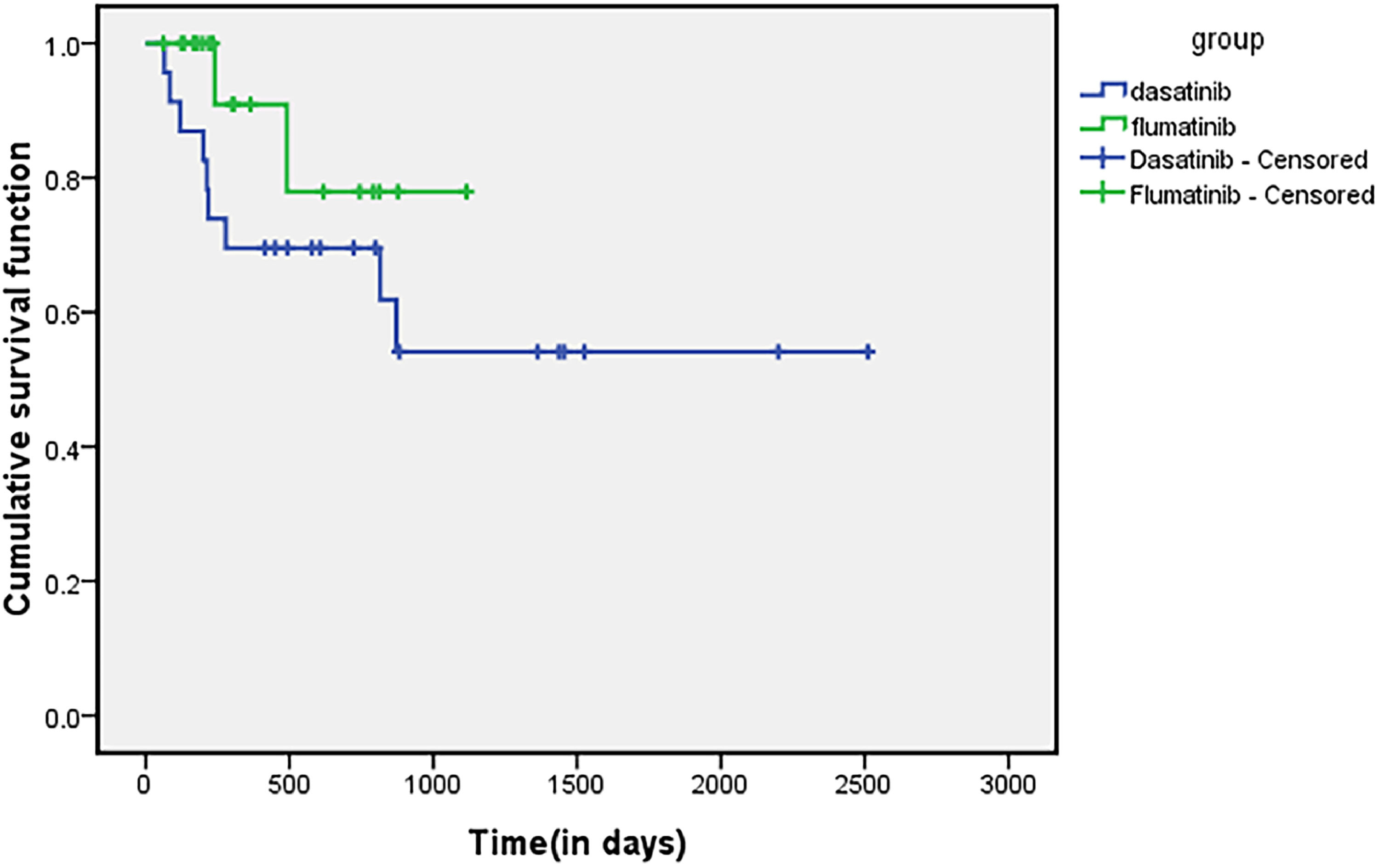
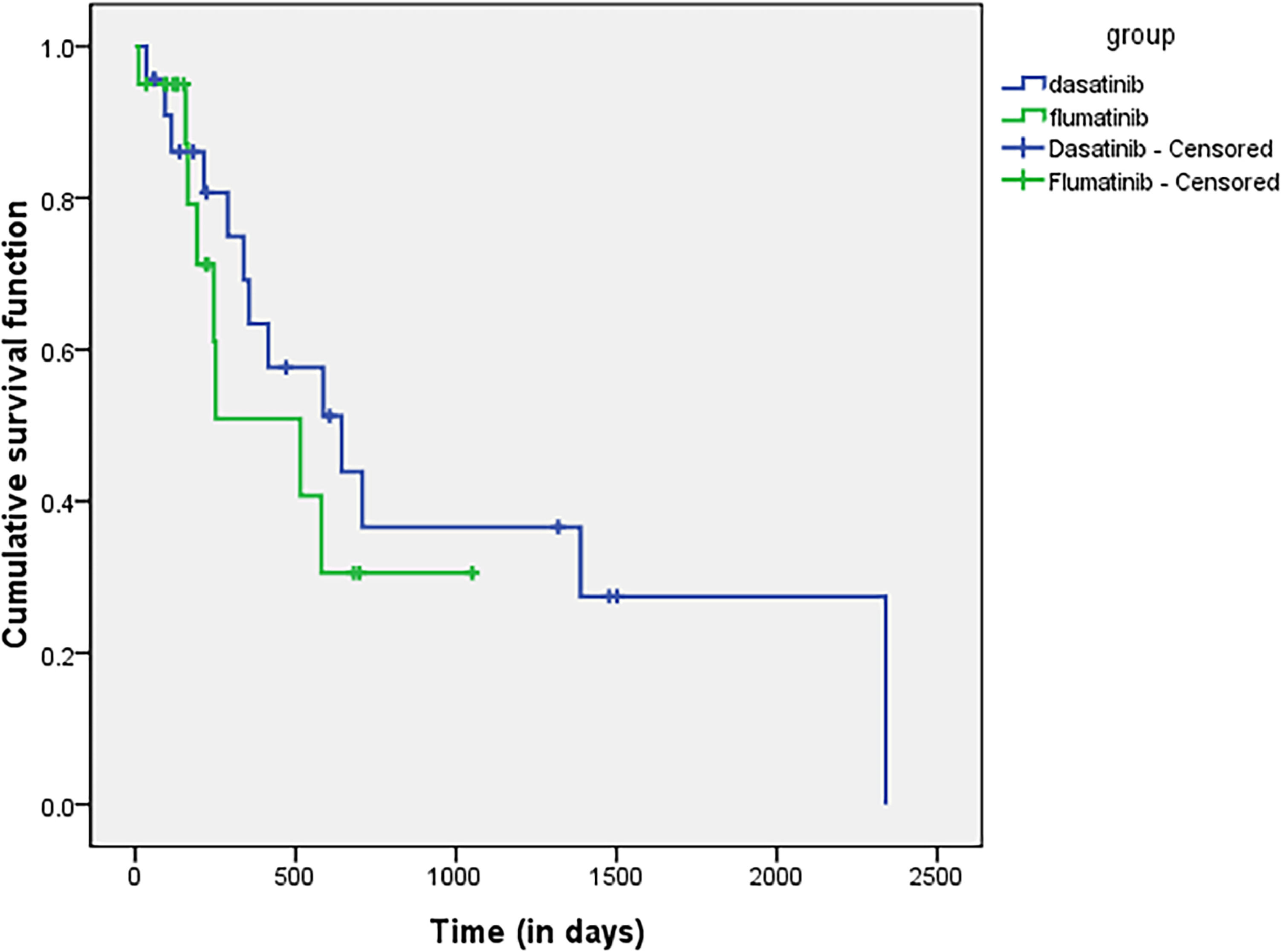
Survival analysisBy the end of the follow-up time, there was no significant difference in OS and PFS between the flumatinib group and the dasatinib group and the 1-year OS and PFS rates in the flumatinib group were 95% and 50.9%, respectively. The 1-year OS and PFS rates of the dasatinib group were 69.6% and 54.5%, respectively. There was no statistically significant difference in the OS (p = 0.113) and PFS (p = 0.243). The survival curves for OS are shown in Figure 3, the survival curves for PFS are shown in Figure 4.
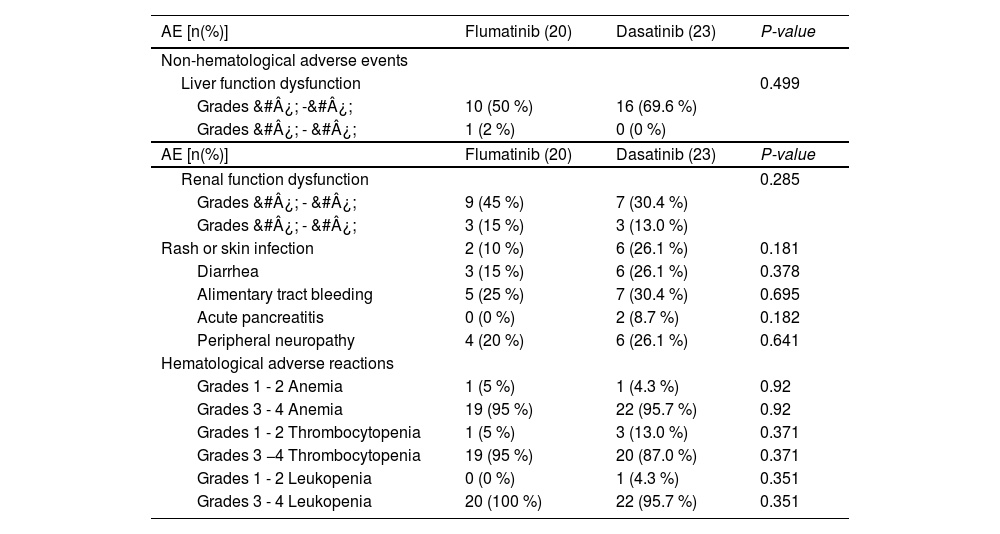
Adverse reactionsThe incidences of hematologic and non-hematologic adverse reactions, including anemia, thrombocytopenia, agranulocytosis, granulocytosis with fever, abnormal liver function, abnormal kidney function, skin lesions, diarrhea, gastrointestinal bleeding, acute pancreatitis, peripheral neuropathy and others were not statistically different between the flumatinib and dasatinib groups (p > 0.05). However, it is noteworthy that two patients in the dasatinib group experienced acute pancreatitis, whereas no cases of pancreatitis were observed in the flumatinib group. Additionally, in the dasatinib group, three patients with difficult-to-control diarrhea experienced relief or improvement after switching to flumatinib. Furthermore, one patient in the dasatinib group was unable to tolerate the treatment due to pleural effusion and binaural mastoiditis. After replacing dasatinib with flumatinib, no related adverse reactions were reported in this patient (Table 4).
Adverse reactions of flumatinib versus dasatinib in patients with Ph+ acute lymphoblastic leukemia.
Flumatinib, a highly selective ABL kinase inhibitor, demonstrates stronger inhibition of intracellular BCR-ABL tyrosine kinase activity, compared to imatinib. In its molecular structure,8 flumatinib has undergone structural modifications by replacing the phenyl ring with a pyridine ring and introducing a trifluoromethyl group. These modifications aim to enhance the binding strength between flumatinib and BCR-ABL1 kinase, leading to increased stability of the binding interaction. In vitro studies have demonstrated that flumatinib exhibits significant inhibitory effects on mutations occurring in the ATP-binding region of ABL kinase, such as the V299L, F317L, and F317I.9 An open, randomized, multicenter phase III clinical trial comparing the safety and efficacy of flumatinib with imatinib in patients with chronic myeloid leukemia (CML-CP) showed a higher, faster and deeper response and a lower incidence of adverse events in patients treated with flumatinib.10 In a clinical study of flumatinib treatment for Ph+ ALL involving 9 newly diagnosed and relapsed patients, a 100% complete response (CR) rate was achieved within 28 days. The study also reported a CMR rate of 44.4% and an MMR rate of 22.2%, with a total CMR rate of 66.7% at 3 months.11 In a retrospective study of flumatinib treatment for Ph+ ALL, 25 patients were evaluated. Among them, 21 patients (84%) received flumatinib during the initial induction phase, while 4 patients (16%) received it after completing the first course of induction therapy. The CR rates at 28 days, 3 months and 6 months were 88%, 91.67% and 90.48%, respectively. The CMR rates were 60%, 75% and 80.95% at the corresponding time points.12
The MRD is the strongest independent predictor of recurrence risk and long-term survival in all ALL age groups.13,14 Nicholas J. Short et al. proved through clinical studies that achieving CMR within 3 months is also an important prognostic factor for ALL patients.15 In this study, the MRD negativity of the flumatinib group was 90% and the CMR rate was 75% within 3 months, while the MRD negativity of the dasatinib group was 56.5% and the CMR rate was 46.2% within 3 months, there being statistical significance between the two groups (p < 0.05). It has been suggested that flumatinib may bring a better long-term prognosis in the treatment of Ph+ ALL. In this study, the median follow-up time of the flumatinib group was 272 (62 – 1116) days and the PFS was 178.5 (11 – 1051) days, while the median follow-up time of the dasatinib group was 606 (66 – 2510) days and the PFS was 338 (35 – 2340) days. The OS and PFS in the dasatinib group were longer than those in the flumatinib group, but there was no statistical significance (p > 0.05), considering that the clinical application of flumatinib in Ph+ ALL patients was later and the follow-up time was shorter. In addition, the sample size is relatively small, so future studies with a larger sample size are needed to confirm the differences in OS and PFS between the two groups.
The TKI combined with chemotherapy and followed by the allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains the standard treatment for Ph+ ALL.16 A clinical retrospective study of 6 patients with Ph+ ALL showed that flumatinib combined with induction chemotherapy and sequential allo-HSCT had good efficacy and safety in the treatment of newly diagnosed Ph+ ALL.17 In this study, 4 patients in the flumatinib group received flumatinib combined induction chemotherapy and sequential autologous hematopoietic stem cell transplantation (auto-HSCT), among whom 3 patients remained progression-free for a long time after transplantation, the median follow-up time being 304 (220 - 744) days. By the end of the follow-up, all 3 patients were disease-free. The fourth relapsed 115 days after hematopoietic stem cell transplantation and was accompanied by mutations in the T315I, SETD2, BLM and MPL, an RET gene germline mutation and a PAX5 gene deletion. Three patients in the dasatinib group received dasatinib combined with induction chemotherapy and followed by HSCT. One patient was replaced with flumatinib after auto-HSCT because he could not tolerate the adverse reactions of dasatinib, but no special discomfort was found at the end of the follow-up. One patient had a complete response followed by sequential allo-HSCT, but relapsed 6 months later. In the other case, autologous hematopoietic stem cell transplantation failed and the treatment was abandoned. In this clinical observation, flumatinib combined with induction chemotherapy and followed by HSCT was superior to dasatinib in the treatment of newly diagnosed Ph+ ALL. However, due to the small number of patients included, no definite conclusion can be drawn, and more samples are needed to compare the differences between the two groups in the future.
It is worth noting that studies have indicated that consolidation with allo-HSCT can improve survival rates in patients with Ph+ ALL upon achieving the initial complete remission (CR1).18 However, a retrospective study conducted by Armin Ghobadi et al. compared the outcomes of Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) patients who achieve CMR within 90 days and received or did not receive allo-HSCT (allo-HSCT: 98; non-HSCT: 132). The results of multivariable analysis and propensity score matching analysis showed that adult patients with Ph+ ALL who achieved CMR within 90 days after initiating treatment did not derive a survival benefit from the allo-HSCT during CR1.19 With the administration of TKIs, the impact of HSCT on survival benefits has gradually diminished.20
Regarding adverse reactions, studies have indicated that flumatinib does not inhibit the c-Src kinase, which is associated with the development of pleural effusion, and the VEGFR2, which is linked to cardiovascular events. Additionally, flumatinib has a weaker inhibitory effect on the c-KIT and PDGFRβ kinase compared to imatinib, resulting in a lower incidence of hematologic toxicity and edema. Compared to other second-generation TKIs, such as dasatinib and nilotinib, flumatinib demonstrates fewer off-target effects and a higher safety profile.8 In this real-world observational study, no significant difference was observed in the incidence of anemia, thrombocytopenia, agranulocytosis, abnormal liver function, abnormal renal function, skin lesions, diarrhea, gastrointestinal bleeding, acute pancreatitis and peripheral neuropathy between the flumatinib group and the dasatinib group. Moreover, no serious adverse events leading to drug interruption or replacement occurred in the flumatinib group. Considering that this study is a retrospective study and the previous medical records are incomplete, the adverse reactions of patients may be more extensive. Further expansion of the sample size is needed to confirm whether there is a difference in adverse reactions between the two groups.
ConclusionFlumatinib has demonstrated the ability to achieve early CMR and MRD negativity in newly diagnosed Ph+ ALL patients. It is well tolerated, with mild adverse reactions in clinical practice, making it a viable treatment option. However, the limited number of clinical cases and follow-up duration in this study necessitate confirmation through prospective multicenter clinical trials to validate these conclusions.