Diversity in Classical Hematology Research
More infoBrazil has many inequities in the healthcare provided nationwide. Therefore, in order to access challenges in treatment, available resources and current practices, to identify barriers in delivering a good quality of care among Brazilian centers treating children and adolescents with Non-Hodgkin Lymphoma (NHL) and to generate a future prospective guideline, a customized online survey was distributed to pediatric hematologists and oncologists across the country.
ResultsA total of 97 surveys were completed (35% response rate), from 47 cities in all Brazilian regions and 79 units of care, with a median of 1 answer by the center (range 1 - 5). Most respondents work at an institution supported exclusively by public/philanthropic resources (58%), with an average of 5 to 9 new cases/year (49%), and 41% have 4 to 6 oncologists/centers. Additionally, 22% have no easy access to the intensive care unit, 26% have no access to Rasburicase, 28% have no access to Rituximabe as front-line therapy and 41% have unreliable methotrexate monitoring levels. Those differences cannot be explained thoroughly by regional wealth variances, nor by the financing model. Regarding the pathology service, 70% consider having reasonable quality assistance, but the timeframe to deliver diagnosis is satisfactory to 46%. There is no uniform management of care, with the current guideline from the Sociedade Brasileira de Oncologia Pediátrica being adopted by 54 to 59%, depending on the NHL subtype.
ConclusionThis study provides insights into the heterogeneity of care among Brazilian centers. Recognizing those diversities will support the design of effective strategies and collaboration nationwide.
Non-Hodgkin Lymphoma (NHL) is a heterogeneous group of diseases and clonal tumors of B cells, T cells or NK cells at different maturation stages. Each subtype differs on the underlying genetic mechanism, cell of origin, pathology, clinical presentation, spreading patterns, sensibility to available drugs and prognosis.[1,2]
The most common childhood and adolescent NHL subtypes are Burkitt lymphoma (BL), diffuse large B cell lymphoma (DLBL), lymphoblastic lymphoma (LL), primary mediastinal B cell lymphoma (PMBL) and anaplastic large cell lymphoma (ALCL).[2]
Over the last decades, several multi-institutional and international cooperative groups in high-income countries (HICs) have established best practices for most childhood and adolescent NHL subtypes, with overall survival (OS) rates exceeding 80 to 90%.[2,3] This incredible success stems from intensive and risk-stratified chemotherapy regimens delivered with low toxic death rates.[3] Moreover, this significant improvement has only been possible through international and collaborative efforts among different HICs, given that this is a rare condition, as are all neoplastic diseases in this population.[2,3]
Despite this tremendous progress reached by the HICs in the treatment of NHL, the same has not been replicated in low- and middle-income countries (LMICs),[4,5] where 90% of the children live and 80% of all new cancer cases in childhood are diagnosed globally.[5,6] In these latter countries, some other factors should be considered in designing effective strategies to reduce mortality related to NHL care: misdiagnosis, lack of diagnosis or delayed diagnosis, abandonment of therapy, excessive toxic deaths and excessive relapses due to inadequate regimens.[5]
As the largest country in South America, Brazil is characterized by widely disparate socioeconomic conditions between cities and regions,[7,8] which could be translated into an unequal distribution of resources and quality of the healthcare system.
Furthermore, how large are the gaps among health care systems in different cities and institutions? What are the resources at each center dedicated to the care of children and adolescents with cancer? What difficulties has each one been facing? What should be done to achieve equality and diminish disparities? As those questions soar, they urge to be answered.
This study aims to report the results of a survey of physicians responsible for the care of children and adolescents with NHL countrywide to understand the daily challenges they face taking care of these children, to learn about the available resources and current practices, to identify barriers, to deliver good quality care and to generate a future prospective guideline for the Brazilian territory.
MethodThis was a cross-sectional study with a descriptive design. An unpaid online questionnaire was delivered to all doctors responsible for caring for children and adolescents with NHL in Brazil, with no exclusion criteria, between January 19 and April 7, 2021. The survey was sent by email and common social media platforms to all registered pediatric oncologists and hematologists, members (active or not) of the Brazilian Society of Pediatric Oncology (SOBOPE).
The online questionnaire was composed of 25 questions (supplementary material). The first part gathered information about the centers, including location (city/state), model of financing, characterized as either 100% public/philanthropic, meaning caring for children covered by the Universal Healthcare System (Sistema Único de Saúde [SUS]) or receiving funds from non-governmental organizations, private institutions that accept exclusively private insurance, or institutions that are a combination of both.
The following questions asked about the maximum age group of patients treated by the center, number of pediatric oncologists and number of NHL cases diagnosed per year.
Questions related to available resources focused on ready access to the pediatric intensive care unit for oncologic patients (e.g., a limited number of beds) and access to Rasburicase for patients with the tumor lysis syndrome. Regarding the perceived quality of the pathology services, the question inquired about: the existence of a referral pathologist with expertise in pediatric cancer/NHL and satisfactory immunohistochemistry panel availability, as well as the timeliness to receive the final pathology reports (less than a week, 1 to 2 weeks or more than two weeks or other), or whether none of the pathology-related questions were satisfactory.
Questions related to therapy were geared towards access to methotrexate serum levels and the maximum methotrexate regimen that could safely be administered at the hospital.
In the second part, information was gathered about the management of the different NHL subtypes, protocols used for each subtype, difficulties faced in applying the treatment (staging, management of initially bulky disease, response evaluation, toxicity management, concern about the final pathology, case discussion and consultation, as well as the treatment of rare cases). Participants were questioned regarding their likelihood of participating in a retrospective registry for NHL, providing a reason for participating (or not) in the Brazilian National Protocol for NHL and the difficulties in registering prospective data for the protocol.
Finally, participants were asked whether or not they would be interested in participating in a prospective trial for mature B-NHL and possible motivation, such as management support, participation in a study group, improvement in the management of these cases, participation in the guideline development group, participation in data gathering for quality improvement and financial support per patient registration.
Among the 277 SOBOPE members (direct communication), the goal was to have representation from all federative units (26 states and the Federal District) with centers dedicated to the care of children and adolescents and at least one answer from each center. Center identification was not required, but desirable.
Ethics approvalThis research was approved by the Ethical Review Board at the Centro Infantil de Investigações Hematológicas Dr. Domingos A. Boldrini (CAAE: 40387220.7.0000.5376) and followed the regulations codified by the Declaration of Helsinki.
Data analysisAll voluntarily answered questions were entered into Excel (Microsoft Excel 2019). Based on previous surveys, we expected a response rate of approximately 30%.
The data collected were mostly categorical and were organized in frequency, absolute and percentual values, median and range. Tables and charts were constructed by Microsoft Excel 2019. Comparisons between groups made use of the chi-square test.
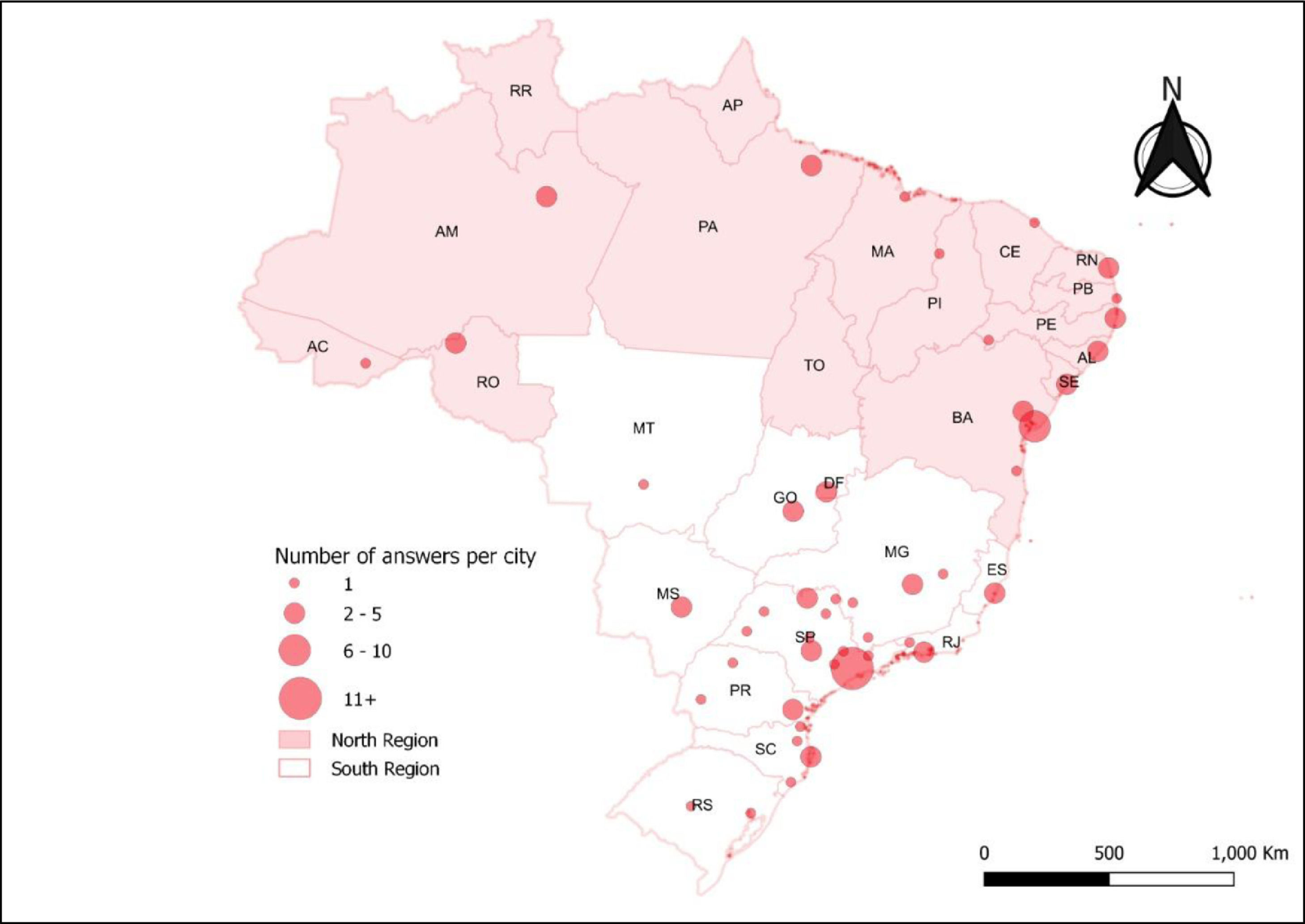
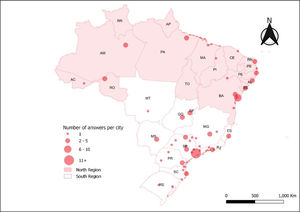
Information about the geographic location also served as a basis for designing a descriptive map, using the ArcGIS 10.4 software, which allowed spatialization of the number of answers per city. Brazilian regions were divided into north and south, the north being less affluent and comprising the north and northeast geopolitical microregions, according to vulnerability.[7,8]
ResultsWe received 97 answered questionnaires (35% of the SOBOPE members), with 24 of the 27 federative units (Figure 1), 47 cities and 79 different centers that treat childhood cancer being represented by the responders and a median of 1 answer per center (range 1 - 5); seven centers were not identified. A total of 65% of the respondents were in the Southern region and 25% of answers came from the State of São Paulo.
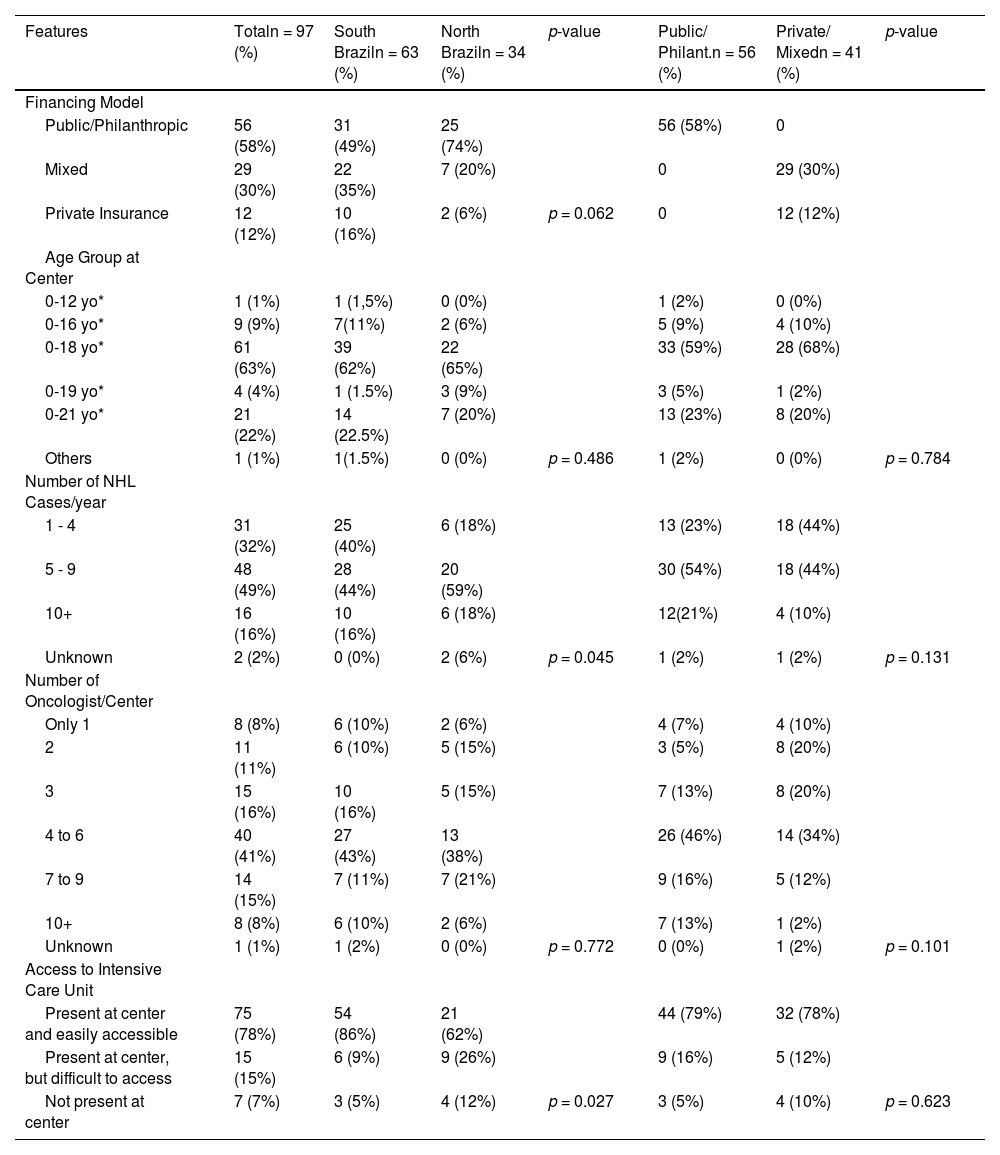
Table 1 describes the characteristics of the centers. The majority of the responders work at a center supported uniquely by public/philanthropic funds (58%), treating children and adolescents under 18 years of age (63%), having an average of 5 to 9 new cases/year (49%) and employing 4 to 6 oncologists per center (41%). Among the answers, 78% have easy access to intensive care units (ICU), whereas 22% have not, and the southern region has significantly (p = 0.027) better access (86%) than the northern region (62%).
Characteristics of surveyed centers. yo*= years old, Philant.= Philanthropic
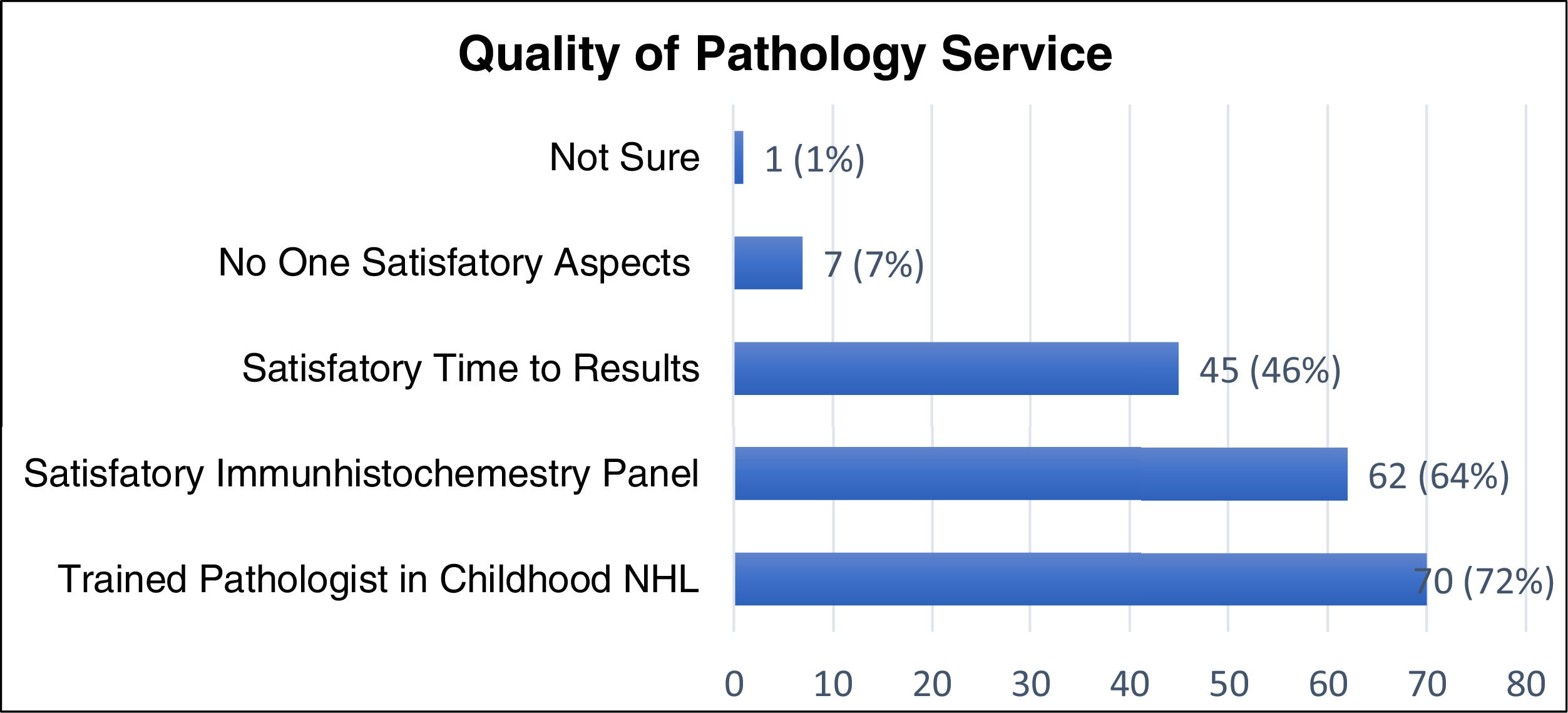
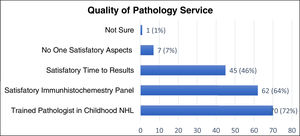
Figure 2 describes the quality of the pathology services. Most responders consider that the reference pathologist has expertise in childhood NHL (72%), with a satisfactory immunohistochemistry panel (64%). However, only 46% perceive the final pathology reports to be given in a timely fashion (up to one week) and 7% feel their access to quality pathology services was inadequate in all aspects.
When assessing the objective timeliness in receiving the final pathology results, 28% of respondents reported receiving it within one week, 55% between one and two weeks, 15% after two weeks and 2% answered with “other.”
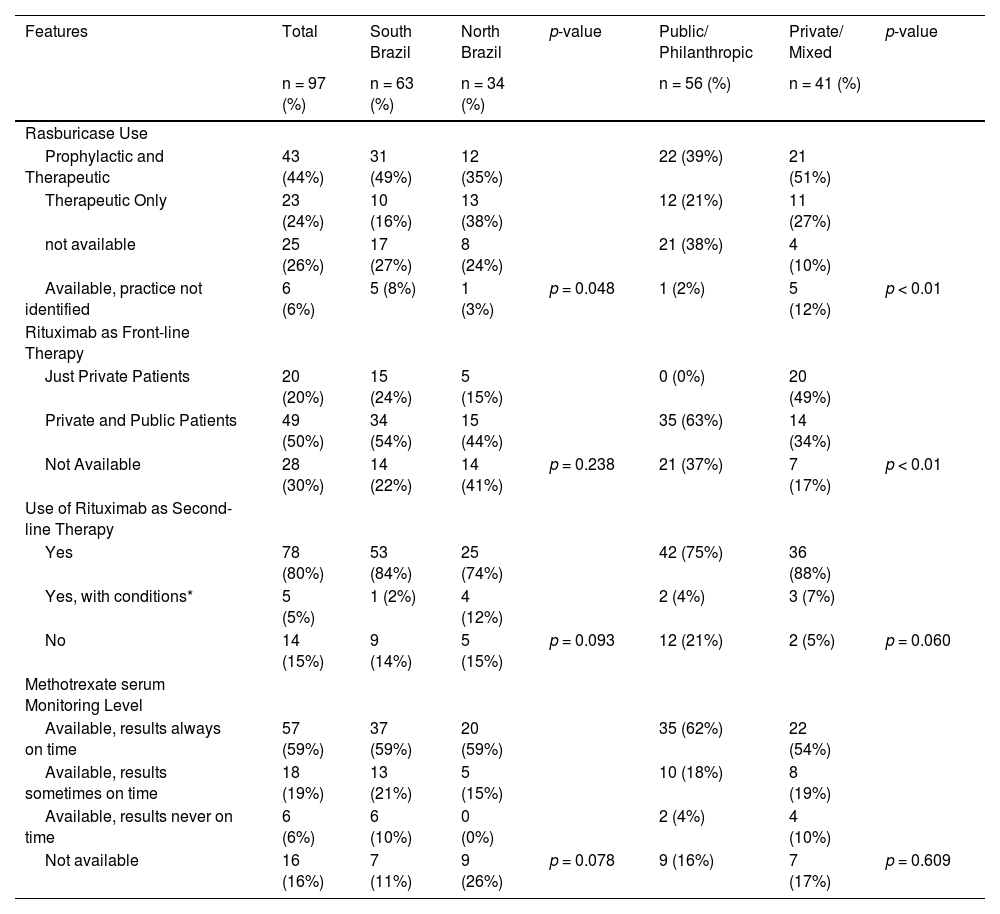
Table 2 describes the accessibility to essential medications, such as Rasburicase and Rituximab, and required evaluations, such as serum methotrexate (MTX) monitoring levels. The latter was available to 59% of the responders, Rasburicase and Rituximab as front-line therapy for high-risk mature B NHL were not available to 26% and 30%, respectively. In contrast, 80% of the respondents have easily acess to Rituximab as a second-line therapy.
Access to essential medications and required exams. * With conditions, e.g., private insurance, financial support by a non-governmental organization.
Analysis by regions separated into north and south showed no significant difference in most aspects, except for the number of new cases per year, access to ICU and the prophylactic use of Rasburicase for tumor lysis, as answers from the southern region more frequently considered its prophylactic use. However, the unavailability of this medication was the same in both regions.
Analysis of the financing model revealed differences only in the access to Rasburicase and Rituximab as front-line therapy, both of them being more available to mixed public/philanthropic and private centers and exclusively private insurance-supported centers.
Concerning the current practice in the choice of the treatment regimens for different NHL subtypes, there were a variety of responses. In general, the majority (59%) follow the current recommendation from the SOBOPE (2016) for mature B NHL (BL and DLBL), 59% for LL, 54% for ALCL and 54% for PMBL. The other alternative regimens listed were only chosen by up to 20% of the respondents, depending on the histologic subtype.
Regarding the difficulties faced in the care of children with NHL from the physician's perspective, the management of rare cases was the most commonly named concern (58%). However, as the items were not mutually exclusive, the distribution of other challenging aspects was the initial clinical support for bulky cases for 30%, management of acute toxicities for 26%, case discussion and consultation for 26% and inadequate quality of the pathology services for 20%, among other rarer reasons.
The majority of the surveyed physicians (95%) consider pivotal a national treatment guideline for children and adolescents with NHL.
DiscussionIn Brasil, twenty-four of the 27 federative units regularly report treating children and adolescents with NHL and have centers that care for cancer patients within those age groups (SOBOPE and direct communication). This survey gathered information from all those 24 federative units and many institutions, with 79 different ones identified, between private insurance, mixed and public-supported centers.
This study highlights the difficulties and differences within the country in treating children and adolescents with NHL, throughout infrastructure, access to essential medications, number of specialized physicians, quality of pathology services and current treatment strategies from the caring physician's perspective. Some easily identified barriers were: the difficult access to intensive care units (22%); medications, such as Rasburicase (26%) and Rituximab as front-line therapy for high-risk B NHL (30%); reliable serum methotrexate monitoring levels (41%), and; finally, an inadequate timeline to receive a final pathology report (54%).
Some resources mentioned above are critical and elementary for the assistance and, allied to the fact that Brazil is an upper-middle-income country, altogether that reveals the lack of a national pediatric cancer strategy plan to address more uniform management, providing some essential capabilities to overcome the absence of local initiative and promoting good quality of care nationwide.
Those differences cannot be exclusively explained by regional wealth variances, with the northern region being the most vulnerable,[7,8] nor can they be explained by the financing model. As the centers organize and establish a reliable funding system, they seem to be able to deliver better assistance, but finding the root of those diversities will be beyond this study's scope.
Brazil has a Universal Healthcare System (SUS) available to all citizens, but it has many deficiencies. In order to bridge those inadequacies, approximately 28% of the Brazilian population, the wealthier group, also has private health insurance.[9] Most respondents (58%) were from centers supported exclusively by public resources, either SUS or a fund-raising organization. While the financial model did not determine the number of oncologists at each center, nor access to intensive care units or serum methotrexate monitoring levels, it was significantly correlated with the access to front-line therapy medications, such as Rasburicase and Rituximab.
Another aspect unveiled by this survey was the number of respondents working alone or with one other oncologists (19%) and in centers diagnosing four or fewer new NHL cases/year (32%), with the southern region having significantly more of those centers (40%) than the northern region (18%). Although the small centers have a role in pediatric cancer care, this aspect could be an obstacle, as it is well recognized that higher volume hospitals, higher case volume providers and specialized pediatric oncology hospitals positively impact survival outcomes.[10,11]
Moreover, concerning some essential medication, such as high-dose methotrexate (HD-MTX), plays a pivotal role in treating NHL, serum MTX levels must be accessible for the safe delivery. Unfortunately, among responders, 41% do not have reliable access and treatment strategies should consider this limitation.
In a context of a highly toxic chemotherapy regimen, as in the treatment of childhood NHL, Rituximab is an attractive agent for mature B NHL. An international randomized clinical trial confirmed its efficacy and safety as a first-line agent added to standard chemotherapy in children and adolescents with high-risk mature B NHL.[12] In addition, this drug could be added to lower-intensity chemotherapy to increase the efficacy without adding substantial toxicity.[13]
Regrettably, the high cost of Rituximab makes it unaffordable in many settings. In Brazil, based on this survey, this medication is not available as first-line therapy to 30% of the respondents, whereas 80% of the answers report access to Rituximab as a rescue therapy, a setting in which relapsed and refractory mature B NHL have a very dismal prognosis.[14]
According to the latest data from a Childhood and Adolescents Cancer Registry in Brazil,[15] 650 new cases of NHL are expected to be diagnosed each year under the age of 19. Nonetheless, the fate of this population is uncertain and best practices need to be designed to consider those Brazilian variances in the healthcare system.
The same Brazilian Cancer Registry publication[15] shows a minimal declining tendency for the age-adjusted mortality related to NHL under the age of 19 during the last 35 years, from a rate of 4 to 2.7 per million in 2013. While for the same period and age group, data from the Surveillance, Epidemiology and End Results (SEER) Program revealed a more pronounced decline in mortality, from a rate of 4.41 to 0.72 per million in 2013.[16]
Nevertheless, a more detailed evaluation of treatment effects and survival for that age group by NHL subtype is still lacking in Brazil. Some scarce literature reporting treatment outcomes at single institutions using treatment regimens locally adapted from protocols developed by cooperative groups in HIC have shown that toxic mortality is higher than that observed in the original protocols.[17,18] Moreover, this study demonstrated there is no uniform practice to treat childhood and adolescents with NHL.
Traditionally, the SOBOPE Brazilian Guidelines to treat childhood and adolescents with NHL were translated from international HIC protocols and, hence, are not feasible at many institutions, making them not widespread.
Regarding mature B NHL, the most frequent subtype and more distinctive management, with an escalation in the intensity of chemotherapeutic regimens according to risk stratification, there is no proven superiority of any treatment strategy compared to others in LMIC and the best regimen varies not only by country, but by the specific center or regional characteristics.[5] In order to design an effective therapy for mature B NHL in those countries, the risk of toxic death against the excess of relapse must be balanced when considering chemotherapy intensification, as mature B NHL is a disease of one treatment, with the outcome for relapsed cases remaining very low, even in HICs.[14]
Understanding the diversity of resources available to Brazilian institutions will aid in the development of specific treatment protocols that are tailored to those different situations. Therefore, national or centralized protocols need to be stratified into different tiers, considering local treatment assets and variance across the country and supported by sound evidence-based strategies.[5,6]
Furthermore, with this tier-adapted guide, many institutions across the country and others throughout Latin American countries could cooperate regardless of their resources, considering that similar successful collaborations have already been established among these countries.[4,19] As more alliances become possible, finding the best strategy for each situation becomes feasible, leveraging research in this area. Unfortunately, insufficient local data, lack of infrastructure for clinical research and funding shortages are barriers to consider in conceiving this guideline.
Limitations of the present study include the possibility of bias selection, as it is probable that only interested and available physicians answered the survey. Additionally, some questions were opinion-based, which may not represent the whole group's opinion at each center. Another limitation is whether we actually identified all the centers that treat children and adolescents in the country.
ConclusionsThis study provides insights into the heterogeneity of resources and care of children and adolescents with NHL at centers across all Brazilian regions. Recognizing those diversities will support the design of effective strategies and collaboration nationwide.
We thank Guilherme Correia for the assistance with the Brazilan map/Figure 1. Furthermore, we are thankful for the support of Maria Lydia Mello de Andréa and the SOBOPE, especially Carolina Pietro and Dr. Cláudio Galvão de Castro Jr. Finally, we want to acknowledge all colleagues for their contribution to the survey and willingness to share their insights.