Follicular and mantle cell lymphoma are low-grade B-cell malignancies that lack good responses to chemoimmunotherapy. This study aimed to assess retrospectively clinicopathological features and to determine independent prognostic factors for follicular and mantle cell lymphoma patients treated at two Brazilian medical centers: the Hematology and Hemotherapy Center of the Universidade Estadual de Campinas (Unicamp), a public university hospital, and AC. Camargo Cancer Center, a specialized cancer center.
MethodsTwo hundred and twenty-seven follicular and 112 mantle cell lymphoma cases were diagnosed between 1999 and 2016. Archived paraffin blocks were retrieved and reviewed. Corresponding demographics and clinical data were recovered from medical charts. Outcome analyses considered both overall and event-free survival.
ResultsFor follicular lymphoma treated with the R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, prednisone) and R-CVP (rituximab, cyclophosphamide, vincristine sulfate, prednisone) regimens, both B-symptoms (p-value<0.01 for overall and event-free survival) and high-risk Follicular Lymphoma International Prognostic Index (p-value<0.01 for overall survival) were independently associated to worse prognosis. Maintenance with rituximab improved the prognosis (p-value<0.01 for overall survival). For mantle cell lymphoma, B-symptoms (p-value=0.03 for overall survival and event-free survival) and bone marrow infiltration (p-value=0.01 for overall survival) independently predicted reduced survival, and rituximab at induction increased both event-free and overall survival (p-value<0.01 in both analyses). Combinations of these deleterious features could identify extremely poor prognostic subgroups. The administration of rituximab was more frequent in the AC. Camargo Cancer Center, which was the institution associated with better overall survival for both neoplasias.
ConclusionThis study represents the largest cohort of follicular and mantle cell lymphoma in South America thus far. Some easily assessable clinical variables were able to predict prognosis and should be considered in low-income centers. In addition, the underuse of rituximab in the Brazilian public health system should be reconsidered in future health policies.
Low-grade non-Hodgkin lymphomas (LG-NHL), also known as indolent lymphomas, encompass a peculiar group of neoplasias, generally characterized by incomplete response to therapy, poor perspective of cure and frequent relapses.1 Histologically, these neoplasms display predominantly small lymphoid cells with condensed chromatin, small quantities of activated cells, a diffuse or nodular architectural pattern and low mitotic activity. The most prevalent subtypes of LG-NHL are B-cell lymphomas: follicular lymphoma (FL; comprising 29% of all NHL), lymphocytic lymphoma (12%), mucosa-associated lymphoid tissue (MALT) lymphoma (9%) and mantle cell lymphoma (MCL; 7%).2
Indolent clinical courses with prolonged survival are expected for all these entities, with the remarkable exception of MCL, which presents with a more aggressive clinical behavior.3 Special attention should be brought to FL (also the main type of LG-NHL among Brazilian patients) and MCL (due to its more aggressive clinical features that often lead to treatment challenges).
Currently, the main first-line therapeutic choices for LG-NHL include an anti-CD20 monoclonal antibody (rituximab) combined with chemotherapy. In spite of greatly improving survival, the inclusion of rituximab seemed not to change the paradigm of incurable disease for LG-NHL.4,5 Complementarily, most LG-NHL cohorts did not reach a sufficiently long follow-up time to observe therapeutic impact on the risk of death, such as that observed for rituximab maintenance in FL.6,7
An increasing number of studies have helped to elucidate both the biology of the neoplastic cells and the composition of the tumor microenvironment in LG-NHL, especially for FL8–12 and, to a lesser extent, for MCL13,14 and lymphocytic lymphoma.15,16 As a result of these investigations, new therapeutic approaches beyond rituximab appeared for LG-NHL, such as lenalidomide (an immunomodulatory imide drug), and ibrutinib (Bruton tyrosine-kinase inhibitor).17–19 Novel therapies introduced a new era in LG-NHL research and treatment, marked by transduction pathway targeting. This scenario stresses the need for studies that address the natural history of these diseases so far, enabling future comparisons in new transitional contexts.
Studies with large groups of LG-NHL patients are lacking in Brazil. Therefore, this study aimed to describe clinical and pathological features, as well as outcomes of patients diagnosed with FL and MCL over the last 17 years in two large hospitals in São Paulo State, Brazil.
MethodsDesign and patientsThis retrospective study included previously untreated patients diagnosed with FL and MCL that were followed-up at the Hematology and Hemotherapy Center of the Universidade Estadual de Campinas (HHC – Unicamp) and at the Medical Oncology service of AC. Camargo Cancer Center (ACCCC) between 1999 and 2016. HHC-Unicamp is a public University Hospital, where all patients are covered by the National Health Service. ACCCC is a non-profit foundation and includes mostly patients from private health insurance companies, but also patients covered by the National Health Service. This study was approved by the local Ethics Committees (CAAE number: 32177014.3.0000.5404), and all research procedures were in accordance with the Declaration of Helsinki.
All cases were classified according to the World Health Organization (WHO) classification for lymphoid tumors.2 Patients with localized cutaneous disease, pediatric follicular lymphoma, and those with histological grade 3B FL were excluded from this study. All patients with insufficient clinical data (e.g. no staging or insufficient follow-up data) were also excluded from the study.
The formalin fixed, paraffin embedded tissues of patients were submitted to hematoxylin and eosin (H&E) staining to evaluate morphology. Immunohistochemical expressions of CD20, CD10 and BCL6 were considered to characterize FL, whereas expressions of CD20, CD5 and cyclin D1 confirmed the diagnosis of MCL. When necessary, a more comprehensive panel was performed.
For cases diagnosed with FL, H&E slides were revised to discriminate histological grade (1, 2 or 3A). For MCL cases, H&E slides were revised and classified for cytological pattern (classical, small cell, pleomorphic, blastoid) and histological pattern of infiltration (diffuse, nodular or mantle zone). Cases with fine-needle biopsies or with an exclusive bone marrow biopsy for diagnosis could not be classified.
Histological transformation (HT) in FL was also assessed, and defined as a new onset of disease after FL treatment, associated with high-grade lymphoma features seen in a new biopsy.
Clinical dataRelevant clinical data were collected from the patient's medical charts, including: age at diagnosis, gender, Ann Arbor staging, presence of B-symptoms, presence of bulky (>7cm) disease, presence of extranodal disease (excluding bone marrow), presence of bone marrow infiltration at diagnosis and first-line therapy regimen, if performed. In addition, the date of diagnosis, date of first relapse (when available) and date of last follow-up were computed.
The prognostic reference scores for FL and MCL [the Follicular Lymphoma International Prognostic Index (FLIPI)20 and the Mantle Cell Lymphoma International prognostic Index (MIPI)21] were also collected when possible. The International Prognostic Index (IPI) was not included in this study, due to its inferior discriminating capacity for survival in FL and MCL. Furthermore, due to limited information on β2-microglobulin at diagnosis, the FLIPI-2 index was not considered.
Response to initial therapy was collected for cases treated with the R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, prednisone) and R-CVP (rituximab, cyclophosphamide, vincristine sulfate, prednisone) regimens and classified as complete or partial response, progressive disease and primary refractory disease, in accordance with the International Working Group Criteria.22 For statistical purposes, complete response rates and also overall response (i.e., complete+partial responses compared to the remaining two) were assessed.
Survival definitionFor all patients, both the event-free survival (EFS) and overall survival (OS) were calculated. The first was defined as time from diagnosis until death due to disease, disease progression or last follow-up. The OS comprised the time from diagnosis until death from any cause or last follow-up. Patients without an event at the last follow-up date were censored. The last update on patient survival was performed on April 2017.
Statistical analysesDescriptive statistics was used initially to address the patients’ features. Association tests included the Chi-squared or Fisher's exact test to compare frequencies. Survival analysis was performed by testing all clinical and pathological variables that were collected. Simultaneous testing of redundant information (e.g. Ann Arbor staging and the FLIPI index) was not performed. Survival curves were plotted using the Kaplan–Meier method, and compared with the log-rank test. Cox univariate regressions were also performed for all variables influencing survival in Kaplan–Meier curves. Finally, a Cox multivariate model was proposed, including all variables with a p-value of less than 0.10 in univariate analysis. For all tests in this study, a p-value<0.05 was considered statistically significant.
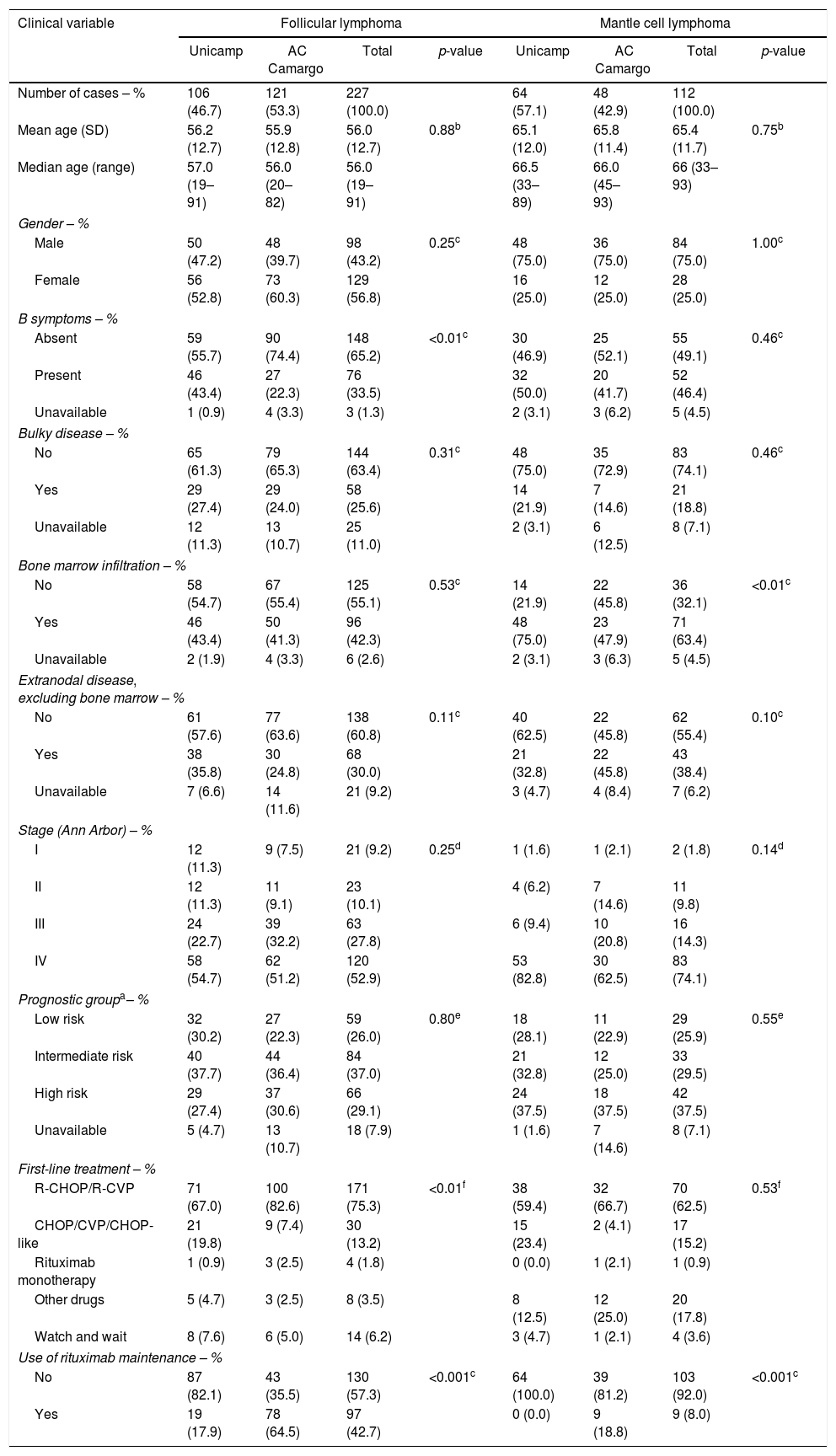
ResultsClinicopathological features and treatmentOf the eligible cases for analysis, 227 were FL and 112 were MCL. One hundred and six of the FL cases (46.7%) were from HHC-Unicamp and 121 (53.3%) were from ACCCC; MCL cases were mostly from HHC-Unicamp (57.1%). Of note, in 14 (12.5%) MCL cases, the diagnosis was made with clinical evaluation associated with cyclin-D1 positivity in bone marrow biopsies only (there was no collection of other tissue sources). The main clinical features of all patients at diagnosis are summarized in Table 1. FL patients had a slight female predominance and they were mostly categorized into the FLIPI intermediate-risk group. MCL patients, on the other hand, were more frequently male and classified as high-risk according to the MIPI index. In addition, both diseases were more likely to present with advanced Ann-Arbor stages (III or IV).
Clinical features of patients with follicular and mantle cell lymphoma in both institutions.
| Clinical variable | Follicular lymphoma | Mantle cell lymphoma | ||||||
|---|---|---|---|---|---|---|---|---|
| Unicamp | AC Camargo | Total | p-value | Unicamp | AC Camargo | Total | p-value | |
| Number of cases – % | 106 (46.7) | 121 (53.3) | 227 (100.0) | 64 (57.1) | 48 (42.9) | 112 (100.0) | ||
| Mean age (SD) | 56.2 (12.7) | 55.9 (12.8) | 56.0 (12.7) | 0.88b | 65.1 (12.0) | 65.8 (11.4) | 65.4 (11.7) | 0.75b |
| Median age (range) | 57.0 (19–91) | 56.0 (20–82) | 56.0 (19–91) | 66.5 (33–89) | 66.0 (45–93) | 66 (33–93) | ||
| Gender – % | ||||||||
| Male | 50 (47.2) | 48 (39.7) | 98 (43.2) | 0.25c | 48 (75.0) | 36 (75.0) | 84 (75.0) | 1.00c |
| Female | 56 (52.8) | 73 (60.3) | 129 (56.8) | 16 (25.0) | 12 (25.0) | 28 (25.0) | ||
| B symptoms – % | ||||||||
| Absent | 59 (55.7) | 90 (74.4) | 148 (65.2) | <0.01c | 30 (46.9) | 25 (52.1) | 55 (49.1) | 0.46c |
| Present | 46 (43.4) | 27 (22.3) | 76 (33.5) | 32 (50.0) | 20 (41.7) | 52 (46.4) | ||
| Unavailable | 1 (0.9) | 4 (3.3) | 3 (1.3) | 2 (3.1) | 3 (6.2) | 5 (4.5) | ||
| Bulky disease – % | ||||||||
| No | 65 (61.3) | 79 (65.3) | 144 (63.4) | 0.31c | 48 (75.0) | 35 (72.9) | 83 (74.1) | 0.46c |
| Yes | 29 (27.4) | 29 (24.0) | 58 (25.6) | 14 (21.9) | 7 (14.6) | 21 (18.8) | ||
| Unavailable | 12 (11.3) | 13 (10.7) | 25 (11.0) | 2 (3.1) | 6 (12.5) | 8 (7.1) | ||
| Bone marrow infiltration – % | ||||||||
| No | 58 (54.7) | 67 (55.4) | 125 (55.1) | 0.53c | 14 (21.9) | 22 (45.8) | 36 (32.1) | <0.01c |
| Yes | 46 (43.4) | 50 (41.3) | 96 (42.3) | 48 (75.0) | 23 (47.9) | 71 (63.4) | ||
| Unavailable | 2 (1.9) | 4 (3.3) | 6 (2.6) | 2 (3.1) | 3 (6.3) | 5 (4.5) | ||
| Extranodal disease, excluding bone marrow – % | ||||||||
| No | 61 (57.6) | 77 (63.6) | 138 (60.8) | 0.11c | 40 (62.5) | 22 (45.8) | 62 (55.4) | 0.10c |
| Yes | 38 (35.8) | 30 (24.8) | 68 (30.0) | 21 (32.8) | 22 (45.8) | 43 (38.4) | ||
| Unavailable | 7 (6.6) | 14 (11.6) | 21 (9.2) | 3 (4.7) | 4 (8.4) | 7 (6.2) | ||
| Stage (Ann Arbor) – % | ||||||||
| I | 12 (11.3) | 9 (7.5) | 21 (9.2) | 0.25d | 1 (1.6) | 1 (2.1) | 2 (1.8) | 0.14d |
| II | 12 (11.3) | 11 (9.1) | 23 (10.1) | 4 (6.2) | 7 (14.6) | 11 (9.8) | ||
| III | 24 (22.7) | 39 (32.2) | 63 (27.8) | 6 (9.4) | 10 (20.8) | 16 (14.3) | ||
| IV | 58 (54.7) | 62 (51.2) | 120 (52.9) | 53 (82.8) | 30 (62.5) | 83 (74.1) | ||
| Prognostic groupa– % | ||||||||
| Low risk | 32 (30.2) | 27 (22.3) | 59 (26.0) | 0.80e | 18 (28.1) | 11 (22.9) | 29 (25.9) | 0.55e |
| Intermediate risk | 40 (37.7) | 44 (36.4) | 84 (37.0) | 21 (32.8) | 12 (25.0) | 33 (29.5) | ||
| High risk | 29 (27.4) | 37 (30.6) | 66 (29.1) | 24 (37.5) | 18 (37.5) | 42 (37.5) | ||
| Unavailable | 5 (4.7) | 13 (10.7) | 18 (7.9) | 1 (1.6) | 7 (14.6) | 8 (7.1) | ||
| First-line treatment – % | ||||||||
| R-CHOP/R-CVP | 71 (67.0) | 100 (82.6) | 171 (75.3) | <0.01f | 38 (59.4) | 32 (66.7) | 70 (62.5) | 0.53f |
| CHOP/CVP/CHOP-like | 21 (19.8) | 9 (7.4) | 30 (13.2) | 15 (23.4) | 2 (4.1) | 17 (15.2) | ||
| Rituximab monotherapy | 1 (0.9) | 3 (2.5) | 4 (1.8) | 0 (0.0) | 1 (2.1) | 1 (0.9) | ||
| Other drugs | 5 (4.7) | 3 (2.5) | 8 (3.5) | 8 (12.5) | 12 (25.0) | 20 (17.8) | ||
| Watch and wait | 8 (7.6) | 6 (5.0) | 14 (6.2) | 3 (4.7) | 1 (2.1) | 4 (3.6) | ||
| Use of rituximab maintenance – % | ||||||||
| No | 87 (82.1) | 43 (35.5) | 130 (57.3) | <0.001c | 64 (100.0) | 39 (81.2) | 103 (92.0) | <0.001c |
| Yes | 19 (17.9) | 78 (64.5) | 97 (42.7) | 0 (0.0) | 9 (18.8) | 9 (8.0) | ||
R: rituximab; CHOP: cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, prednisone; CVP: cyclophosphamide, vincristine sulfate, prednisone.
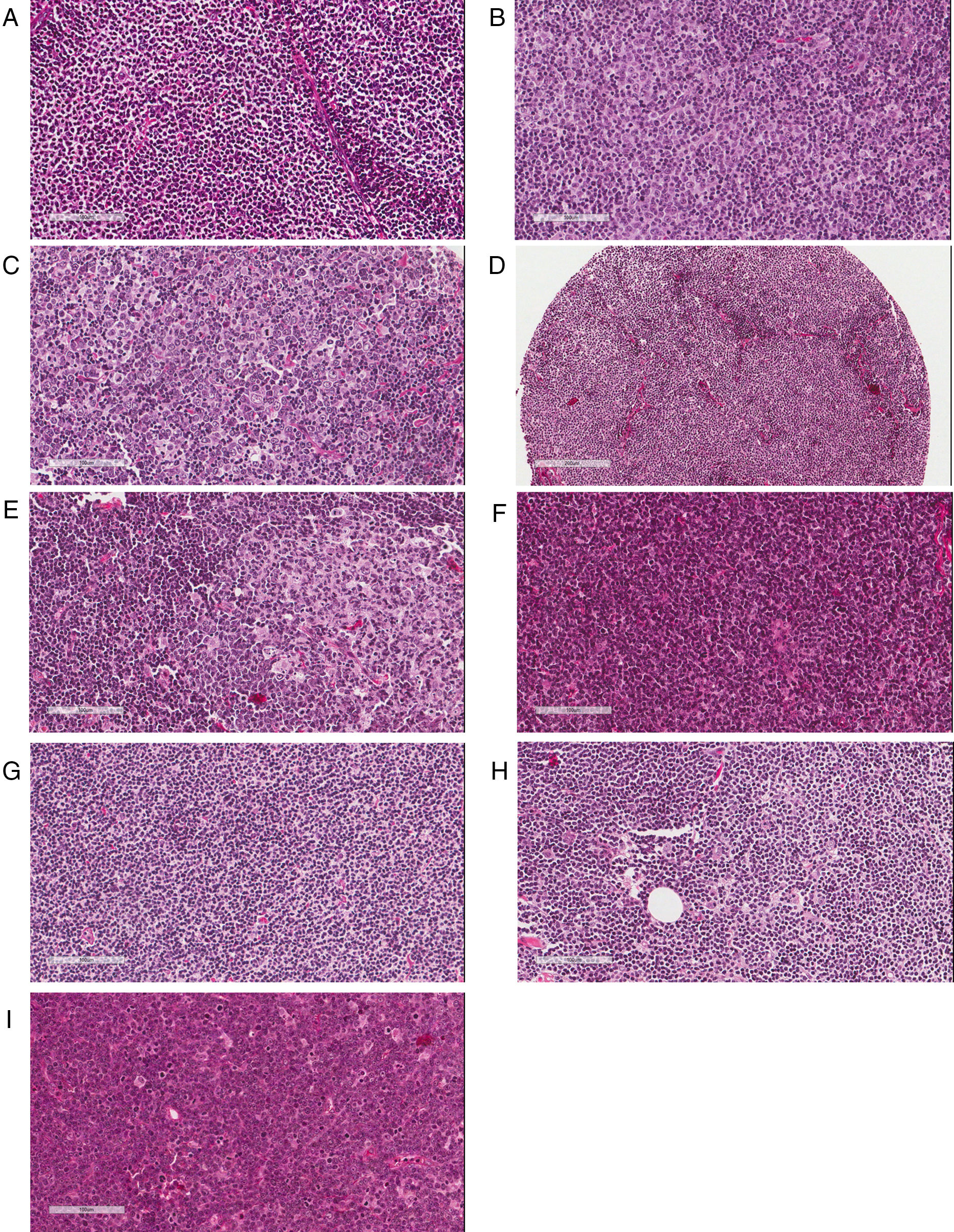
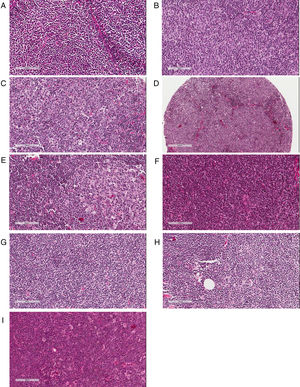
The majority of FL cases was classified as grade 1/2 (n=160 or 70.5%); 49 (21.6%) were graded as 3A. Histological grade could not be determined in 18 cases (7.9%). Regarding MCL patients, the most frequent cytological pattern was classical MCL (n=70 or 62.5%), followed by small-cell (n=15 or 13.4%) and blastoid (n=10 or 9.0%); in 17 cases (15.1%) it was not possible to obtain this information. Additionally, 62 cases (55.4%) of MCL had a diffuse proliferation pattern, followed by 14 (12.5%) with a nodular growth, and three (2.7%) with a mantle zone architecture pattern. In 33 cases (29.4%) this parameter could not be assessed. Artifacts in morphology preservation, fragmentation of the specimen, small fragment or an exclusive bone marrow biopsy were reasons to preclude proper morphological evaluation. Representative examples of histological classifications are shown in Fig. 1.
Pathological assessment of follicular lymphoma (FL) and mantle cell lymphoma (MCL). (A–C) Histological grading of FL: Grades 1 (A), 2 (B) and 3A (C) showing increasing percentages of centroblasts in comparison with centrocytes. (D–F) Patterns of MCL proliferation: nodular (D), mantle zone (E) and diffuse (F). (G–I) Cytological variants of MCL: classic (G), small cell (H) and blastoid (I).
The distribution of clinical features of patients from both institutions was similar. However, FL cases treated at HHC-Unicamp had a higher prevalence of B-symptoms (p-value<0.01), whereas a higher rate of bone marrow infiltration was seen in MCL patients in the same institution (p-value<0.01; Table 1).
The most frequent treatment choice for both diseases was R-CHOP or R-CVP (Table 1). R-CHOP was given to 106 FL patients and 64 MCL cases, whereas R-CVP was prescribed to 65 FL and six MCL patients. Fourteen FL patients did not receive any initial treatment because of low tumor burden. One of these patients progressed with lymph node enlargement after 66 months of follow-up and required treatment at that time (rituximab and chlorambucil). In MCL, four patients were left untreated: in two cases because of an indolent clinical behavior (watch and wait approach), and in the other two due to poor performance status (palliative approach).
The main regimens accounting for ‘other drugs’ in the current cohort (Table 1) were HyperCVAD (cyclophosphamide, vincristine sulfate, doxorubicin hydrochloride, dexamethasone, methotrexate, cytarabine) or Leukeran-based regimens for MCL (four and three cases, respectively). In FL, this category accounted for more diverse therapeutic options, such as R-ICE (rituximab, ifosfamide, carboplatin, etoposide phosphate), gemcitabin, leukeran and DHAP [dexamethasone, high-dose cytarabine (ARA-C) and cisplatin].
The number of patients who received rituximab together with any first-line induction therapy was 179 (78.8%) for FL and 80 (71.4%) for MCL (p-value=0.12 – Chi-squared test). The use of maintenance therapy with rituximab was more prevalent among FL patients (42.7% versus 8.0% when compared to MCL; p-value<0.001 – Chi-squared test). For both groups of patients, maintenance therapy was more frequent after induction regimens with R-CHOP/R-CVP (95 of 97 cases of FL and 7 of 8 cases of MCL).
Follicular lymphoma patients from ACCCC were more frequently administered R-CHOP/R-CVP as first-line regimens compared to those treated at the HHC-Unicamp (p-value<0.01, Table 1); this difference was not detected for MCL (p-value=0.53). Rituximab maintenance was more frequently administered at ACCCC than at HHC-Unicamp for both FL and MCL (p-value<0.001 for both comparisons; Table 1).
No differences were found in complete response on comparing patients treated with R-CHOP and R-CVP both for FL and MCL (p-value=0.56 and 0.67, respectively; Fisher's exact test). For overall response rates, a similar scenario was found (p-value=0.75 and 0.58, respectively for FL and MCL). Due to these results, for further survival analyses (unless otherwise specified), FL patients treated with R-CHOP or R-CVP (171 cases) were grouped together. In MCL, patients who received any chemotherapy or chemoimmunotherapy were included due to the small sample size.
Outcome featuresTransformation to high-grade lymphoma occurred in 15 (6.6%) patients with FL. Median time to transformation was 38.8 months (range: 9.9–175.9 months). Eleven out of 15 patients (73.3%) received R-CHOP/R-CVP as first-line therapy. The remaining four patients (26.7%) received CHOP/CVP. Patients who transformed were initially classified as high-risk disease in eight cases (61.5%), intermediate-risk in four (23.1%), and low-risk in two (15.4%). In one case, FLIPI was not classifiable.
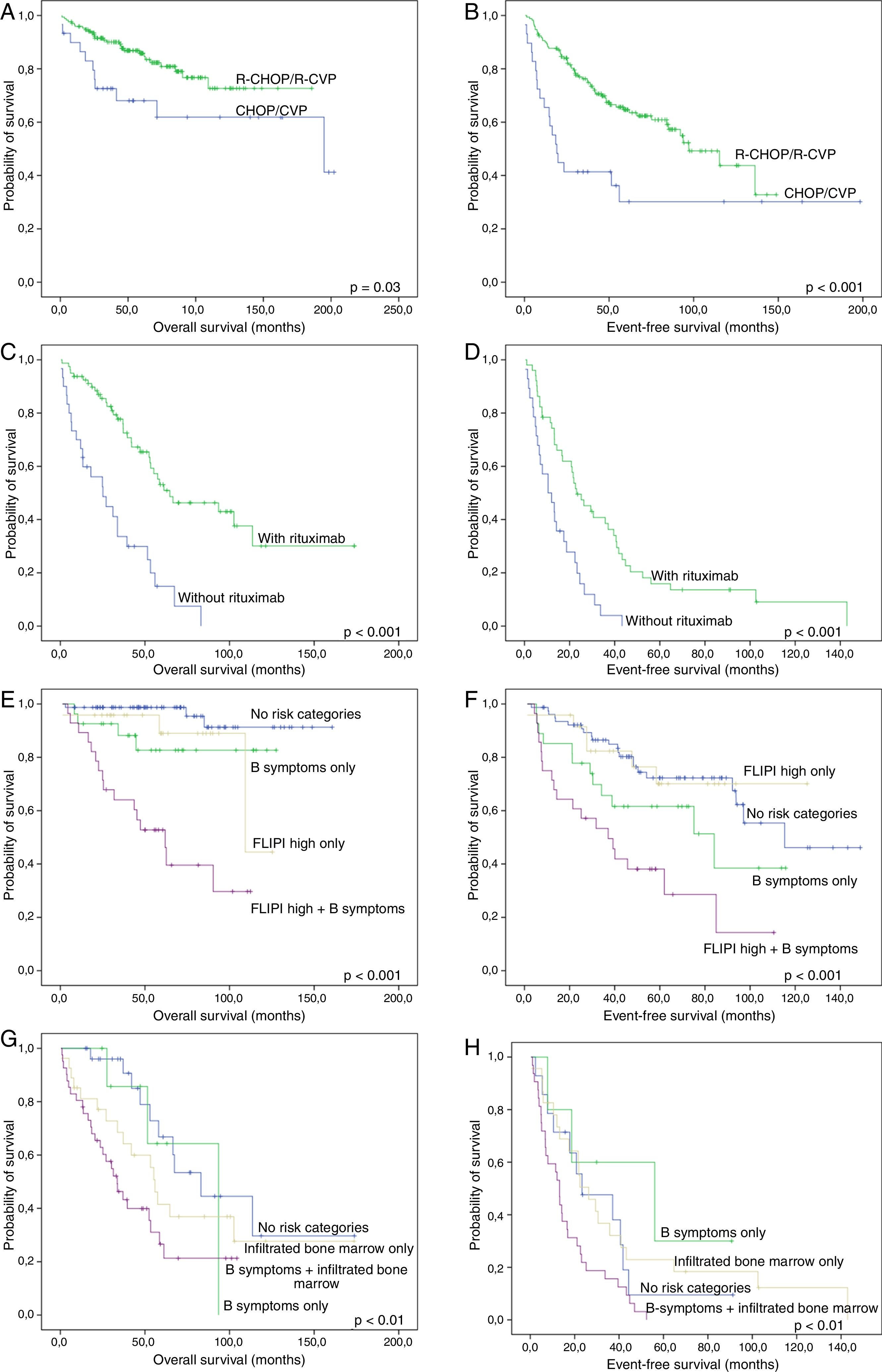
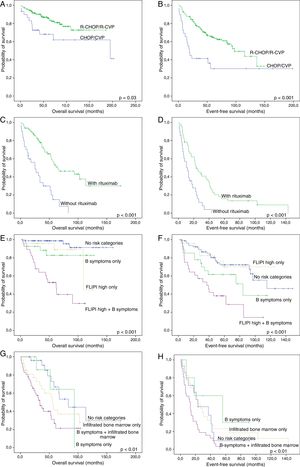
Median follow-up time for FL cases treated with R-CHOP or R-CVP was 55.5 months for all patients (range: 1.3–185.7) and 59.7 months for living patients (range: 8.1–185.7). The figures for MCL were 35.0 (range: 1.0–173.8) and 46.4 months (range: 8.1–173.8), respectively. As expected, FL patients treated with R-CHOP/R-CVP had more favorable OS and EFS than those treated with CHOP or CVP (Fig. 2A and B). Similarly, MCL patients who received anti-CD20 during induction regimens had a more favorable survival (Fig. 2C and D).
Survival curves of follicular lymphoma (FL) and mantle cell lymphoma (MCL) patients. (A) and (B): Overall (A) and event-free (B) survival of FL cases treated with R-CHOP/R-CVP, compared with CHOP/CVP-treated patients. (C) and (D): Overall (C) and event-free (D) survival of MCL patients, grouped according to rituximab administration at induction therapy. (E) and (F): Overall (E) and event-free (F) survival of FL cases treated with R-CHOP/R-CVP, according to the presence of B-symptoms and/or high-risk FLIPI. (G) and (H): Overall (G) and event-free (H) survival of MCL treated with any drug regimen, grouped based on the presence of B-symptoms and/or bone marrow infiltration. All p-values were obtained using log-rank statistics.
In univariate analyses, histological features (i.e., cytological grade for FL, and cytological category and architecture pattern for MCL) did not affect either the OS or EFS.
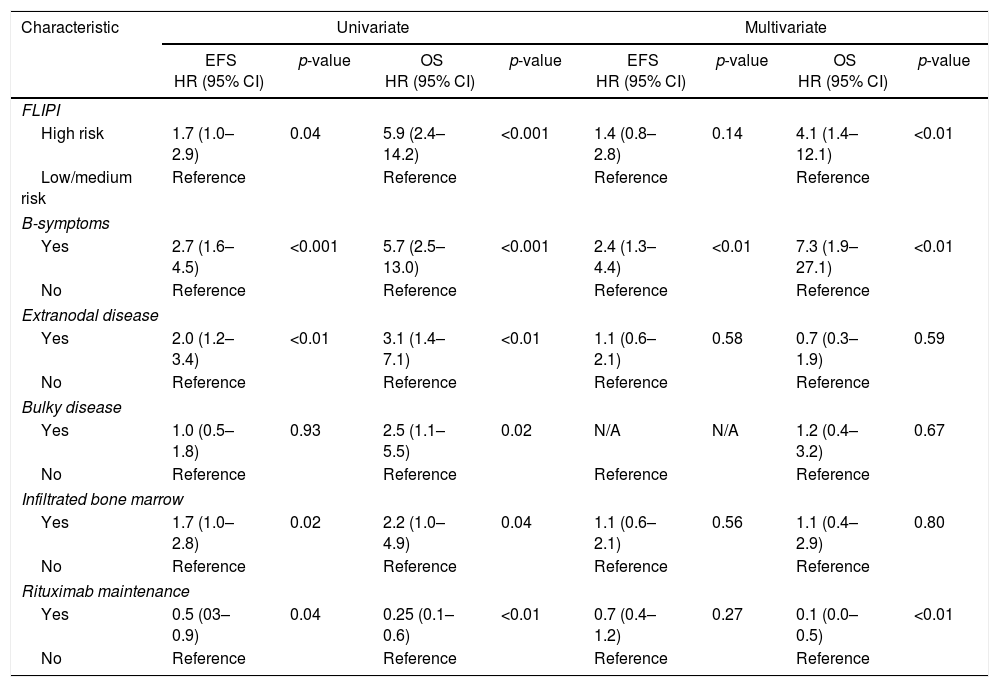
For FL treated with R-CHOP/R-CVP, five clinical variables at diagnosis were related to unfavorable EFS and/or OS: presence of B-symptoms, extranodal disease, infiltrated bone marrow, bulky disease and high-risk FLIPI index. To minimize biases, the use of rituximab as maintenance therapy was also included in the model. Multivariate analyses showed independent adverse values for B-symptoms (both for EFS and OS) and high-risk FLIPI (for OS), as well as a protective role of the use of rituximab maintenance (for OS) (Table 2).
Clinical model developed for patients with follicular lymphoma treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, prednisone) and R-CVP (rituximab, cyclophosphamide, vincristine sulfate, prednisone) regimens.
| Characteristic | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| EFS HR (95% CI) | p-value | OS HR (95% CI) | p-value | EFS HR (95% CI) | p-value | OS HR (95% CI) | p-value | |
| FLIPI | ||||||||
| High risk | 1.7 (1.0–2.9) | 0.04 | 5.9 (2.4–14.2) | <0.001 | 1.4 (0.8–2.8) | 0.14 | 4.1 (1.4–12.1) | <0.01 |
| Low/medium risk | Reference | Reference | Reference | Reference | ||||
| B-symptoms | ||||||||
| Yes | 2.7 (1.6–4.5) | <0.001 | 5.7 (2.5–13.0) | <0.001 | 2.4 (1.3–4.4) | <0.01 | 7.3 (1.9–27.1) | <0.01 |
| No | Reference | Reference | Reference | Reference | ||||
| Extranodal disease | ||||||||
| Yes | 2.0 (1.2–3.4) | <0.01 | 3.1 (1.4–7.1) | <0.01 | 1.1 (0.6–2.1) | 0.58 | 0.7 (0.3–1.9) | 0.59 |
| No | Reference | Reference | Reference | Reference | ||||
| Bulky disease | ||||||||
| Yes | 1.0 (0.5–1.8) | 0.93 | 2.5 (1.1–5.5) | 0.02 | N/A | N/A | 1.2 (0.4–3.2) | 0.67 |
| No | Reference | Reference | Reference | Reference | ||||
| Infiltrated bone marrow | ||||||||
| Yes | 1.7 (1.0–2.8) | 0.02 | 2.2 (1.0–4.9) | 0.04 | 1.1 (0.6–2.1) | 0.56 | 1.1 (0.4–2.9) | 0.80 |
| No | Reference | Reference | Reference | Reference | ||||
| Rituximab maintenance | ||||||||
| Yes | 0.5 (03–0.9) | 0.04 | 0.25 (0.1–0.6) | <0.01 | 0.7 (0.4–1.2) | 0.27 | 0.1 (0.0–0.5) | <0.01 |
| No | Reference | Reference | Reference | Reference | ||||
EFS: event-free survival; OS: overall survival; HR: hazard ratio; 95% CI: 95% confidence interval; FLIPI: Follicular Lymphoma International Prognostic Index; N/A: not applicable.
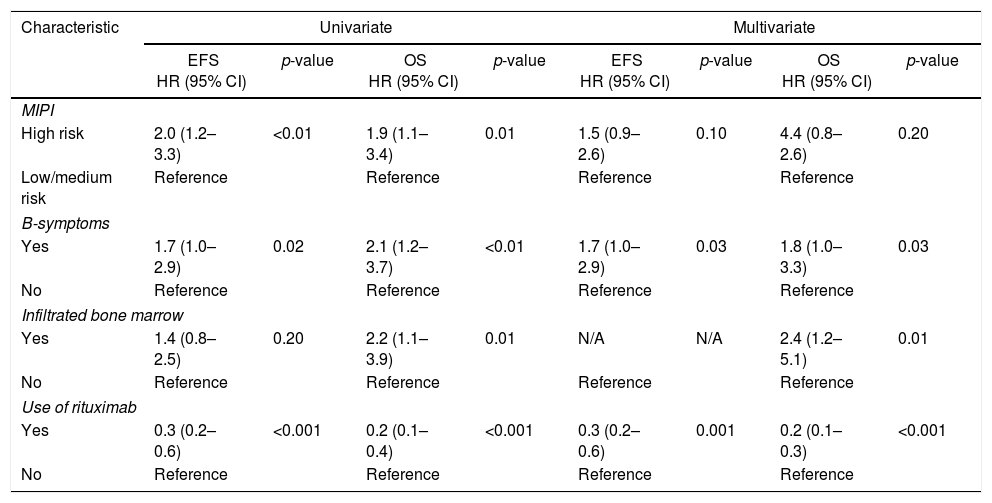
For MCL, univariate survival analyses demonstrated adverse impacts of B-symptoms, infiltrated bone marrow at diagnosis and high-risk MIPI on the EFS or OS. The use of rituximab during induction treatment was included in the model, to reduce therapy-related biases. Joint assessment of all variables in multivariate Cox regression showed independent adverse impacts of B-symptoms (for both EFS and OS), bone marrow infiltration (for OS) and a protective role with the use of rituximab during induction therapy (for both EFS and OS). Interestingly, MIPI lost its prognostic value in the multivariate analysis (Table 3).
Clinical model developed for patients with mantle cell lymphoma treated with any drug regimen.
| Characteristic | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| EFS HR (95% CI) | p-value | OS HR (95% CI) | p-value | EFS HR (95% CI) | p-value | OS HR (95% CI) | p-value | |
| MIPI | ||||||||
| High risk | 2.0 (1.2–3.3) | <0.01 | 1.9 (1.1–3.4) | 0.01 | 1.5 (0.9–2.6) | 0.10 | 4.4 (0.8–2.6) | 0.20 |
| Low/medium risk | Reference | Reference | Reference | Reference | ||||
| B-symptoms | ||||||||
| Yes | 1.7 (1.0–2.9) | 0.02 | 2.1 (1.2–3.7) | <0.01 | 1.7 (1.0–2.9) | 0.03 | 1.8 (1.0–3.3) | 0.03 |
| No | Reference | Reference | Reference | Reference | ||||
| Infiltrated bone marrow | ||||||||
| Yes | 1.4 (0.8–2.5) | 0.20 | 2.2 (1.1–3.9) | 0.01 | N/A | N/A | 2.4 (1.2–5.1) | 0.01 |
| No | Reference | Reference | Reference | Reference | ||||
| Use of rituximab | ||||||||
| Yes | 0.3 (0.2–0.6) | <0.001 | 0.2 (0.1–0.4) | <0.001 | 0.3 (0.2–0.6) | 0.001 | 0.2 (0.1–0.3) | <0.001 |
| No | Reference | Reference | Reference | Reference | ||||
EFS: event-free survival; OS: overall survival; HR: hazard ratio; 95% CI: 95% confidence interval; MIPI: Mantle Cell Lymphoma International Prognostic Index; N/A: not applicable.
In addition, FL patients who were treated with R-CHOP/R-CVP were grouped according to the presence of adverse prognostic factors at diagnosis, i.e., B-symptoms and high-risk FLIPI. Four groups were created: no adverse factors, high-risk FLIPI only, B-symptoms only and the presence of both variables. With this approach, we found a subset of patients with an extremely poor OS and EFS when both high-risk FLIPI and B-symptoms were present (Fig. 2E and F). A similar scenario, especially regarding OS, occurred when grouping together MCL cases with both an infiltrated bone marrow and B-symptoms at diagnosis (Fig. 2G and H).
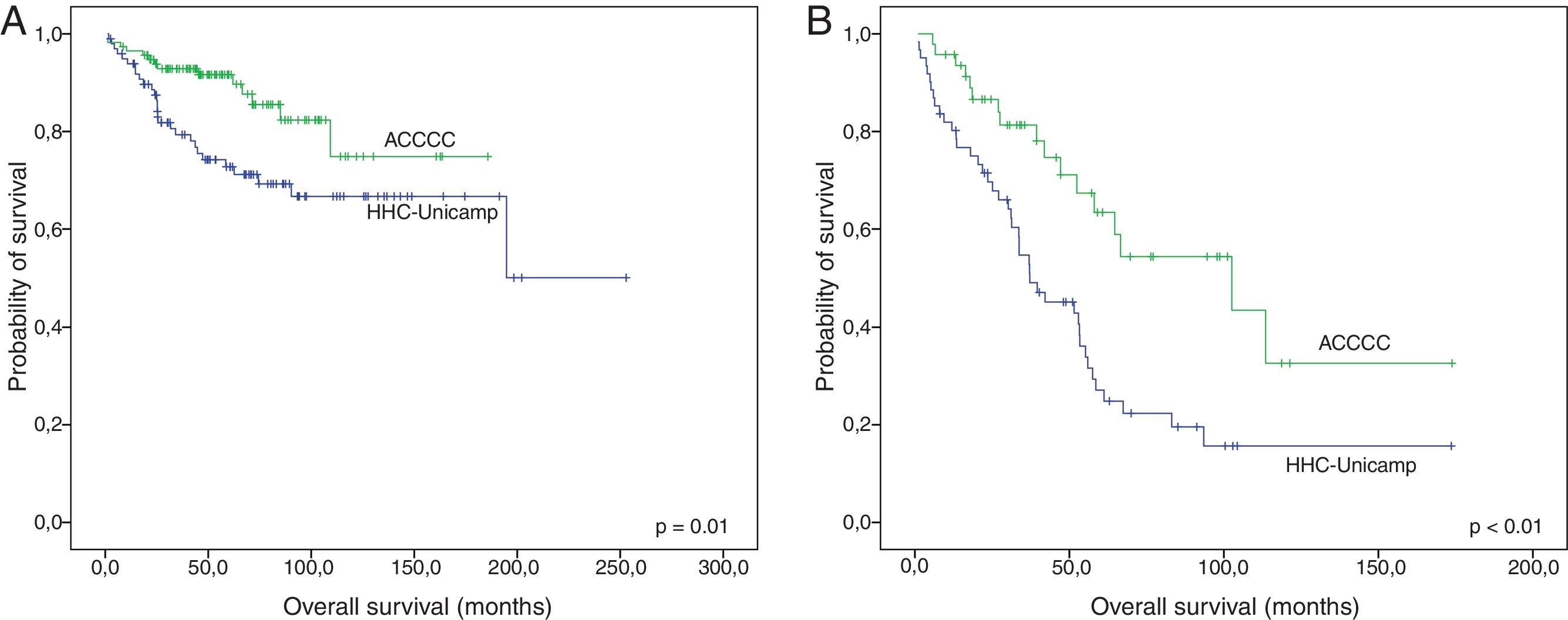
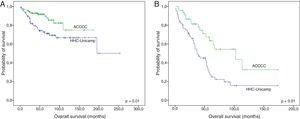
Finally, survival was compared between the institutions in which patients were treated, excluding only the cases that did not receive any drug regimen. Both FL and MCL patients that were treated at HHC-Unicamp presented inferior OS than those treated at ACCCC (p-value=0.01 and 0.001, respectively) (Fig. 3A and B). No differences in EFS were observed (data not shown).
DiscussionThis study is the first one to jointly assess clinical and pathological features of a relatively large cohort of FL and MCL patients treated in South America, the largest reported so far. The presence of B-symptoms and the FLIPI score were highly associated with survival for FL patients, whereas B-symptoms and bone marrow infiltration segregated prognostic groups for MCL. This impact might extend also to other populations, because, in spite of the long time interval considered herein, the majority of these patients were exposed to rituximab. In this setting, the importance of anti-CD20 therapy for these NHLs was stressed, because specific patients receiving no or less rituximab (a scenario more frequently seen in HHC-Unicamp), experienced poorer outcomes.
Some studies in other countries retrospectively addressed features of both FL and MCL. Here, we found a slightly higher proportion of FL cases with B-symptoms (33.5%), compared with two studies conducted in Europe in which B-symptoms were observed in 18.0% and 20.8%.10,23 A higher prevalence of bulky disease was also found in the FL patients of this study (25.6% vs. 14.0% in a cohort from Spain).23 The current cases were also less frequently classified as low-risk according to the FLIPI index compared to another retrospective cohort from Slovenia (26.0% vs. 44.9%,).24 Regarding MCL, the cases of this study had a slightly higher prevalence of B-symptoms than patients in a large European cohort (46.4% vs. 40.8%), however Brazilian cases presented less bone marrow infiltration (63.4% vs. 71.8%).25 Moreover, the prevalence of high-risk MIPI cases described in these patients (37.5%) was not especially different to the rate reported for a North-American cohort (36.2%).26 Taken together, these comparisons show that FL cases from the present cohort seem to present with clinical features associated with higher tumor burden at diagnosis, whereas for MCL, this pattern was not clearly observed.
Patients from the two participating hospitals presented relatively uniform clinical features; however, FL patients at HHC-Unicamp had more B-symptoms, and MCL cases at the same institution had a higher rate of bone marrow infiltration at diagnosis. Interestingly, both variables were associated with poor prognoses of the respective lymphomas. One reason for these differences might reside in faster medical assistance being provided to patients treated by private health care. Patients at ACCCC probably managed to be diagnosed in an earlier stage, with a less symptomatic disease, whereas individuals at HHC-Unicamp (a public hospital) may have had frequent delays in proper medical attention, leading to the diagnosis of lymphomas with greater tumor burdens. However, some environmental (e.g. smoking, exposure to pesticides) and educational (awareness and self-recognition of symptoms) factors might have accounted for the observed differences as well.
Considering treatment differences, FL patients followed at ACCCC were more likely to receive rituximab as maintenance therapy. This is very likely explained by the different institutional profiles: a larger proportion of patients at ACCCC had private healthcare insurance, allowing easier access to this treatment. On the other hand, at HHC-Unicamp, as in other Brazilian public hospitals, rituximab maintenance was included for FL only from 2013 on. Besides, the process to obtain the drug takes longer in the public system. Even considering only FL patients uniformly treated with R-CHOP or R-CVP at induction, the difference between hospital profiles clearly affected survival, as maintenance therapy with rituximab was an important factor to reduce risk of death.
The administration of R-CHOP/R-CVP for MCL occurred in similar proportions at both institutions. The expected benefit of the addition of rituximab at induction for MCL was confirmed5 and, in this case, it did not depend on the hospital in which patients were treated. The role of rituximab maintenance of MCL patients in this study, on the other hand, could not be properly explored due to the small number of patients who received this modality of treatment. However, the few MCL cases receiving anti-CD20 as maintenance were all from ACCCC, and there is already evidence supporting its benefit on patient survival.27
Despite all the differences described, some clinical features considered typical for these two LG-NHL were also seen in the current study, such as a slight female predominance in FL and a higher frequency of male patients in MCL (male:female ratio=3:1). Similarly, the majority of the cases of both lymphomas were staged as III or IV, reflecting indolent courses that lead to the diagnosis usually at advanced stages. Pathological assessment of these NHL corroborated the high prevalence of the ‘classical’ cytological pattern in MCL. One of the largest retrospective studies on MCL described 87.5% of classical cases,25 compared to 62.5% in the present cohort. The blastoid cytologic pattern was less frequent, as expected: less than 10%, mirroring what has been observed by other authors.25 Similar to Tiemann et al.,25 this study also found that the majority of MCL biopsies had a diffuse proliferation pattern. Concerning FL histological grade, these cases were similar to those described in the literature, with grade 3A being less frequent than grades 1/2. However, the impact of discriminating these three grades has been challenged, as all of them experience an indolent course, and similar survival rates. The major difference seems to exist for grade 3B FL (excluded from this study), characterized by more aggressive clinical behavior and inferior outcomes.28
This study, however, presents some limitations inherent to its retrospective design. One of them is the different materials available for diagnosis, for example, bone marrow and core biopsies, which might preclude adequate evaluations of the architecture as mentioned above. Some missing clinical and laboratorial data at first diagnosis and differences in therapy must also be taken into account. Nevertheless, all cases had reliable immunohistochemical evaluations allowing adequate diagnoses of lymphoma subtypes.
A survival model which proved reliable in our FL patients was composed of the FLIPI score (for OS), the use of rituximab maintenance (for OS), and B-symptoms (for both EFS and OS). Maintenance therapy lost its prognostic importance for EFS after adjusting for other clinical variables. However, the influence of rituximab maintenance on OS remained significant, probably reflecting the longer follow-up in the present study, compared to others (such as the prospective PRIMA study with a mean follow-up of 36 months).7 In addition, the presence of B-symptoms was our strongest individual predictor of outcome in FL cases treated with R-CHOP or R-CVP. The combination of FLIPI and B-symptoms refined survival curves even more, revealing a particularly unfavorable prognostic group. Some relevant prognostic scores prior to the FLIPI (e.g. ILI and IFLPFP) incorporated B-symptoms.29 The reintroduction of B-symptoms in new official scores will depend on future validations of their role in larger prospective cohorts of rituximab-treated FL patients.
Previous studies showed a role of HT on FL outcome.30,31 However, we opted not to include this variable in our survival models to avoid bias, since HT is a time-dependent event and the transformation rate of 6.6% was somewhat lower than that reported by Farinha et al. (20.8%)30 and Conconi et al. (13%),31 suggesting an underestimation of HT rates in the present study. This can be possibly due to our median follow-up time, which is shorter than ten years. Therefore, more time is needed to reassess HT in these patients in future studies.
Outcome in MCL was independently associated with the presence of B-symptoms and the use of rituximab at induction (for both EFS and OS) and by bone marrow infiltration at diagnosis (only for EFS). The combination of B-symptoms and positive bone marrow infiltration provided a distinctive prognostic refinement, suggesting a negative impact of high-tumor burden (especially for OS). This finding is novel and encouraging in a disease that lacks consistent prognostic markers. Nevertheless, validation of these results in external cohorts of this rare lymphoma is imperative. After all, the only prognostic variable validated so far for MCL is the proliferative signature, besides MIPI.21,25,32
The fact that the MIPI index lost its prognostic role in the MCL cases of this study by multivariate Cox analyses underscores the need for additional prognostic indexes for this type of lymphoma. In this direction, incorporation of the Ki-67 score on the original MIPI has been proposed for MCL, with promising results.21 A specific study addressing the value of proliferation markers is being prepared by our group. In addition, contradicting previous evidence,25 no influence of MCL cytological variants and histological pattern were found on survival. This may be explained, in part, by differences in treatment, such as a relatively high prevalence of rituximab use in the current cohort compared to historical series.25 However, the limited sample size in the present study might also account for this divergence.
ConclusionsThis relatively large cohort of FL and MCL showed that B-symptoms and the FLIPI score remain highly predictive of survival for FL, whereas B-symptoms and bone marrow infiltration are able to segregate prognostic groups for MCL. We believe that due to the simplicity to assess these prognostic variables, they merit evaluation in future studies on prognostic indexes. Such readily accessible variables are particularly important in the management of FL and MCL in low-income countries. Importantly, we also demonstrated differences in the OS of FL and MCL patients considering two hospital profiles in Brazil. These discrepancies can be explained not only by distinct clinical features of patients, but also by different rituximab administration rates (a modifiable factor). Therefore, the availability of rituximab therapy for these NHL patients, both at induction and for maintenance, should be pursued by current and future public health policies.
FundingThis work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP – grant #2014/09854-5).
Conflict of interestThe authors declare no conflicts of interest.