Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare form of hematological neoplasm with an overall incidence of 0.44%.1 Earlier categorized under acute myeloid leukemia (AML) and related precursor neoplasms in the 2008 World Health Organization (WHO) classification, it is currently recognized as a separate entity within the acute myeloid neoplasms and acute leukemias.2 It was previously known as blastic NK cell lymphoma or agranular CD4+NK cell leukemia/lymphoma due to lack of knowledge of its histogenesis. It is now known to arise from precursors of plasmacytoid dendritic cells (pDCs), hence the name.3 Most patients are elderly, with a median age at diagnosis of 61–67 years, but it can occur at any age, the male:female ratio being 3.3:1. Sites of involvement include skin (60–100%), followed by bone marrow and peripheral blood (60–90%) and lymph nodes (40–50%).2 We present a 28-year-old male with multiple skin lesions for one year followed by lymph node and marrow involvement, which is a typical presentation of this disease.
Case detailsA 28-year-old male presented to our hospital with elevated skin nodules on the shoulder and forearm for one year (Figure 1a). He had a past history of a leg ulcer which had been operated on twice at another institution in the previous year. Reports were not available. The bone marrow examination done at another laboratory was normal. On examination, there were multiple, purplish, elevated subcutaneous nodules on the shoulder joint, forearm and anterior abdominal wall, the largest measuring about 3.5×2.0cm.
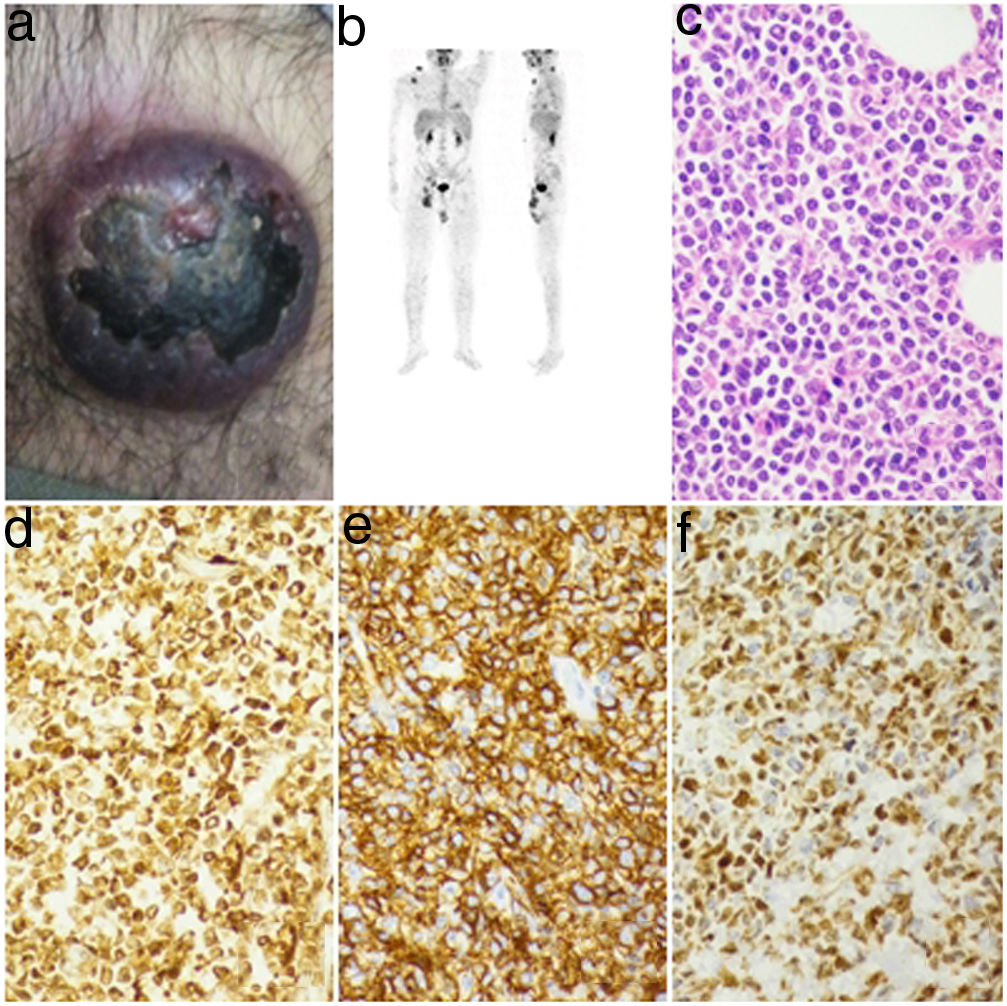
(a) A purplish nodule in right shoulder region. (b) PET-CT image with multiple FDG-avid subcutaneous nodules and enlarged lymph nodes. (c) The cells have a scant amount of cytoplasm with round-to-cleaved nuclei. Mitosis was frequent (H&E 40×). (d) Diffuse and strong cytoplasmic positivity for CD4 (40×). (e) Diffuse and strong positivity for CD56 (40×). (f) Moderate nuclear positivity in tumor cells for TdT (40×).
The laboratory results revealed a hemoglobin level of 15.6gm/dL, total leucocyte count of 5.5×109/L and platelet count of 173×109/L. The initial peripheral smear showed no atypical cells. Liver function and renal function tests were within normal limits. The positron emission tomography contrast tomography (PET-CT) scan revealed faintly fluorodeoxyglucose (FDG)-avid subcutaneous nodules in the right lower limb with post-radiotherapy changes anterior to the right tibia. There were FDG-avid (SUV max 3.2) enlarged right inguinal, right pelvic, retroperitoneal and cervical lymph nodes. Multiple FDG-avid (SUV max 3.1) enhancing soft tissue densities were observed in the right shoulder joint region. Similar FDG-avid subcutaneous nodules were observed in the thorax, anterior abdominal wall and forearm (Figure 1b). A forearm nodule biopsy was performed.
The skin lesion biopsy grossly measured 5.5×3.5×3.5cm. A pigmented area measuring 2.2×1.8cm was seen on the skin surface. The cut section showed a grey white lesion measuring 3.5×3.0cm. The microscopy showed an unremarkable epidermis. There were diffuse sheets of undifferentiated small-to-medium sized round monomorphic cells in the dermis. The nuclei had irregular contours with inconspicuous nucleoli. The cytoplasm was scant and agranular. Mitosis were 18–20/10 HPF. The cells were seen infiltrating into the subcutaneous tissues (Figure 1c). There were areas of coagulative necrosis. However, no angiocentric or angiodestructive growth pattern were seen. The differential diagnosis included leukemia cutis or lymphoma infiltrates.
On performing immunohistochemistry (IHC), tumor cells were diffusely positive for the Leber congenital amaurosis (LCA), CD4, CD7 and CD43, with a strong positivity for CD56. There was heterogenous nuclear positivity for terminal deoxynucleotidyl transferase (TdT) in about 60% of the cells (Figure 1d–f). Cytoplasmic dot positivity for CD68 was noted. Cells were immunonegative for CD34, MPO, CD3, CD2, CD5, CD79a, PanCK (AE1/AE3) and CK20 and Ki-67 was 50%. The BPDCN was diagnosed. A peripheral smear examination was performed after a week, which revealed few atypical lymphoid cells/blasts. Bone marrow aspirate revealed 57% blasts of medium size with round-to-oval nuclei, fine chromatin, one-to-several nucleoli and scant agranular basophilic cytoplasm. There were diffuse infiltrates of small-to-medium sized round immature cells in the bone marrow biopsy. Flow cytometry was not performed due to financial constraints. Cerebrospinal fluid smears were acellular and did not show any atypical cells. The patient was kept on a hyper cyclophosphamide, vincristine, doxorubicin and dexamethasone (CVAD) – Parts A & B regimen. After two cycles of chemotherapy, the skin lesions disappeared and the bone marrow became normocellular. The patient refused any further treatment and after six months the skin lesions returned and the marrow was again involved. The patient was again kept on the hyper CVAD regimen, but this time he did not respond and died after two months due to disease progression.
DiscussionThe BPDCN was reported for the first time in 1994.4 The BPDCN usually presents with skin lesions, regional lymphadenopathy, leucocytosis or thrombocytopenia. Skin lesions can appear as bruise-like or erythematous papules, plaques or tumors up to 10cm. These commonly involves face, trunk and extremities. Lee et al. reported two cases with unusual extracutaneous lesions and reviewed 39 published cases without skin involvement.5 The spleen, liver, tonsil and central nervous system (CNS) can also be involved. It mostly involves elderly, but can occur in any age group, including pediatric patients.6 Our case is a 28-year-old with multiple elevated nodules on the skin and lymphadenopathy. Although our patient has had a one-year history of illness, he did not have any cytopenias at the time of presentation.
The differential diagnosis in the present case includes B-lymphoblastic leukemia (CD34+/−, CD79a+, Tdt+), T-lymphoblastic leukemia (CD34+/−, CD3+, CD2+, Tdt+), myeloid leukemia cutis (MPO+, CD34+), extranodal NK/T cell lymphoma, nasal type (CD3+, CD4−, CD7−, CD56+, CD2+, CD5) and cutaneous T-cell lymphoma (CD3+, Tdt−, CD4/CD8 variable). The present diagnosis was based on clinical findings of multiple skin nodules and the immunophenotypic profile of CD4 and CD56 expression by Tdt+, CD45+ malignant cells that do not express myeloid, lymphoid B or T lineage markers. The plasmocytoid dendritic cell (PDC) associated antigens CD123, CD303, TCL1A, CD2AP, SPIB and TCF4 were not performed due to unavailability. Although, most cases of BPDCN are diagnosed by skin biopsy, patients who have leukemic presentation are diagnosed by flow cytometry. Garnache-Ottou et al. proposed a scoring system for diagnosing BPDCN on flow-cytometry in bone marrow samples.7 They found the BDCA2/CD303 to be the most specific marker for BPDCN. However, in a study of 22 cases of BPDCN, Tsagarakis et al. concluded that a diagnosis of BPDCN can be safely made on flow cytometry with an aim of achieving CD4, CD56, HLADR and CD123 high expression on the CD45 dim population.8
The clinical course is aggressive, with a median survival of 12–14 months.9 Most cases (80–90%) show an initial response to multi-agent chemotherapy (ALL-type, AML-type or lymphoma type), but relapses with subsequent resistance to drugs are regularly observed. Our patient had a good response in skin lesions and attained marrow remission the first time but developed resistance after relapse. He underwent surgery twice before coming to our hospital, which could have been avoided if diagnosis had been made earlier. Allogeneic hematopoietic stem cell transplant (AHCT), especially if offered in the first remission, may result in longer remissions. The current recommendation is to evaluate for an AHCT as soon as possible. The SL-401 is a novel biological-targeted therapy, which targets the cell surface protein CD123 and is being evaluated for the treatment of these patients.10
To conclude, BPDCN is a rare type of hematodermic malignancy with an aggressive course and a poor prognosis. Differentiation from other hematological malignancies is mandatory due to varying treatments and prognoses.
Conflict of interestThe authors declare no conflicts of interest.