Intraoperative blood salvage (cell saver technique) in cardiac surgery is universally used in surgical procedures with a marked risk of blood loss. The primary objectives of this study were to determine the concentration of residual heparin in the final product that is reinfused into the patient in the operating room and to evaluate the efficacy and safety of the cell saver technique.
MethodTwelve patients undergoing elective cardiac surgery were enrolled in this study. Using the XTRA Autotransfusion System, blood samples were collected from the cardiotomy reservoir, both prior to blood processing (pre-sample) and after it, directly from the bag with processed product (post-sample). Hematocrit and hemoglobin levels, the protein, albumin and residual heparin concentrations, hemolysis index, and the platelet, erythrocyte and leukocyte counts were measured.
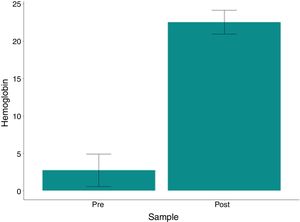
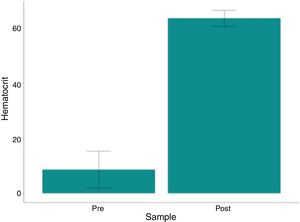
ResultsHematocrit and hemoglobin levels and red blood cell counts were higher in post-processing samples, with a mean variation of 54.78%, 19.81g/dl and 6.84×106/mm3, respectively (p<0.001). The mean hematocrit of the processed bag was 63.49 g/dl (range: 57.2–67.5). The residual heparin levels were ≤0.1IU/ml in all post-treatment analyses (p=0.003). No related adverse events were observed.
ConclusionThe reduced residual heparin values (≤0.1IU/ml) in processed blood found in this study are extremely important, as they are consistent with the American Association of Blood Banks guidelines, which establish target values below 0.5IU/ml. The procedure was effective, safe and compliant with legal requirements and the available international literature.
One of the major challenges threatening patient health and prognosis is the development of a "hemorrhagic condition". Whether developed in clinical patients during a surgical procedure or via trauma, the intensity of the hemorrhagic condition is of significant concern because it is directly proportional to the need to replace the lost blood elements, sometimes immediately, to prevent the condition from becoming irreversible and fatal. The decision to undertake allogeneic blood transfusion should be made in a rational manner, by assessing the relative risks and benefits. The practice of blood transfusion saved countless lives long before complications from this treatment were recognized,1 including viral infections2,3 (e.g., HIV, Hepatitis B and C), parasitic infections (e.g., malaria, T. cruzi, and babesiosis), bacterial infections,4 immunological5,6 and anaphylactic7 reactions, transfusion-related acute lung injury (TRALI)8 and post-transfusion purpura,9 among others.
When considering alternatives to avoid allogeneic transfusions, it was quickly determined that the ideal procedure would be to recover the patient's own blood and immediately reinfuse it. This autotransfusion concept is not new, as indicated by many reports in the literature, starting in 1818 with Blundell10 and subsequently with Highmore11 in animal experiments. Duncan made the first report in the medical literature of his experience in reinfusing a patient's own blood that was lost during surgery.12 A U.S. Air Force surgeon, Dr. Klebanoff, developed a simple autotransfusion device based on cardiopulmonary bypass equipment,13 later marketed as the Bentley ATS 100, due to the lack of blood during the Vietnam War. This system has been widely used, although several reports of complications have been described. In the early 1970s, engineer Allen “Jack” Latham developed a new form of cell salvage.14 He developed the Latham bowl, which had the ability to wash the blood before it was re-transfused. Washing salvaged blood reduced most of the debris or eliminated many of the previously encountered side effects. There were two major stimuli for the development and improvement of this system: (1) cardiac bypass surgery, which in the 1970s went from a radical procedure in advanced high-risk cases to the most performed surgery worldwide, thus requiring large stocks of blood for treatment of hemorrhagic cases,15 and; (2) the appearance in the 1980s of the acquired immunodeficiency syndrome (AIDS), which was more intense and shocking. The enormous fear of HIV transmission made it necessary to search for a safer and more effective alternative approach, which came in the form of autologous transfusion programs.16 Since then, intraoperative blood salvage (the cell saver technique) has been utilized in cardiac surgery, and numerous automations in operation have been introduced through pre-established protocols to enhance safety, such as the bubble detector, balance, microaggregate filter, panels with audible, visual and interactive alarms and sensors that monitor the leuko-platelet layer, the quality of the supernatant from the washing performed and the hematocrit/hemoglobin concentration levels, modernizing intraoperative blood salvage systems. Although we have already standardized the use of the cell saver technique at our service, the introduction of this new equipment necessitated additional research to determine its safety, efficacy and, especially, the concentration of residual heparin in the final product that is reinfused into the patient, which may significantly alter the clinical risk for intra- or post-operative hemorrhage. These are the main objectives of this study.
MethodologyA prospective, single-center, non-randomized, observational study was performed, with the primary outcome assessing the residual heparin concentration in the final product obtained by an automated blood cell salvage (cell saver) system, as well as its clinical evaluation and efficacy, as secondary outcomes. The study was approved by the Teaching and Research Institute of Hospital do Coração (Instituto de Ensino e Pesquisa at the Hospital do Coração - IEP-HCor), the Syrian Sanatorium Association, São Paulo, Brazil and the National Research Ethics Commission (Comissão Nacional de Ética em Pesquisa - CONEP), under the Certificate of Presentation for Ethical Consideration (CAAE) no. 62937216.6.0000.0060. Signed informed consent was obtained from all participants, in accordance with the rules of the Research Ethics Committee. All aspects of the surgery, blood collection, transfusion and any other relevant concerns were explained to the patients by one of the investigators. The null hypothesis of the study was that the reinfused blood contained a clinically significant concentration of heparin. For the purposes of this study, the authors have adopted the American Association of Blood Banks (AABB) definition of "clinically insignificant" as a threshold concentration of residual heparin <0.5IU/ml.36
PatientsTwelve patients admitted to our institution for cardiac surgery over a period of 12 months agreed to participate in the study. Male or female patients aged 18 years or older who were in a non-emergency situation were eligible. The exclusion criteria included patients who were using warfarin, heparin, or other systemic anticoagulant medications prior to surgery, patients who are prevented from receiving blood and blood components due to religious or spiritual beliefs (e.g., Jehovah's Witnesses), patients with congenital or acquired platelet, red blood cell or coagulation disorders, patients with ongoing or recurrent systemic sepsis and those who were unable to provide full informed consent to participate in the study. Clinical outcome data were collected prospectively as part of our patient screening and analysis system. The patient and surgical characteristics are provided in Table 1.
Patient characteristics and type of surgery.
| Study group (n:12) | Percentage (%) | |
|---|---|---|
| Age (years) | 60.2 (44/73) | – |
| <70 years | 10 | 83.3 |
| Weight (kg) | 79 (57/114) | – |
| Male | 8 | 66.7 |
| Female | 4 | 33.3 |
| Personal history: | ||
| Antiplatelet use | 8 | 66.7 |
| Systemic arterial hypertension | 9 | 75.0 |
| Acute myocardial infarction | 2 | 16.6 |
| Dyslipidemia | 3 | 25.0 |
| Reoperation | 3 | 25.0 |
| Type of surgery: | ||
| Aortic aneurysm correction | 3 | 25 |
| Myocardial Revas. | 3 | 25 |
| Myocardial Revas.+Aneurysmectomy | 1 | 8.3 |
| Valvular Replacement | 5 | 41.7 |
| Hemoglobin: | Mean (min/max) | Standard deviation |
| Pre-operative | 11.9 (8.6/15.3) | 1.92 |
| Immediate post-operative | 11.4 (9.1/14.8) | 1.70 |
| Control 5th day | 9.4 (7.2/12.5) | 1.74 |
| Hematocrit: | ||
| Pre-operative | 36.5 (26.8/44.7) | 5.3 |
| Immediate post-operative | 36.1 (28.1/45.0) | 5.6 |
| Control 5th day | 29.6 (22.2/35.7) | 4.4 |
| Mean times: | ||
| Surgery (h) | 5.6 (3/10) | 1.8 |
| Perfusion (min) | 150.8 (70/415) | 95.1 |
| Anoxia (min) | 80.8 (49/119) | 27.2 |
| Blood loss in the operating room (mean/ml) | 1684 (209/7,536) | – |
The automated blood salvage device used was the XTRA Autotransfusion System with a pulsed flow cellular washing system. Standard operating procedures were followed according to the manufacturer's recommended protocols. Adequately trained operators performed the blood salvage process. All aspirated blood was collected from the time of incision of the skin to the wound closure by a double-lumen suction catheter specific for blood collection. The vacuum suction pressure was maintained up to 100mmHg to minimize hemolysis during aspiration. Anticoagulation of the blood recovered in the cardiotomy reservoir was achieved using a solution of 25,000IU of heparin in 1000ml of 0.9% saline. The anticoagulant flow was adjusted according to the bleeding rate of the surgical field, and this blood was filtered and stored in the reservoir. In addition to blood aspirated from the surgical field, whenever possible, any blood remaining in the extracorporeal circulation circuit was also directed to the reservoir to salvage all autologous blood from the patient. The cell salvage system can be used in manual or automatic operation mode. Automatic operation was prioritized, which allows predetermined standardized blood processing, with minimal operator intervention. The separation of the salvaged blood components mainly depends on the balance among the densities of the various blood components. Because the red blood cells are heavier than the other blood components, they stay close to the walls in the filling phase, whereas the smaller and lighter particles stay toward the center and are forced out so that the red blood cells fill the bowl. The red blood cells are washed and resuspended in 0.9% saline, resulting in a hematocrit value of approximately 60%. These washed autologous red blood cells are transported to a sterile reinfusion bag and transfused to the patient intraoperatively. In the perioperative period, all patients received leuko-depleted and homologous red blood cell concentrate as blood replacement therapy. In patients with excessive blood loss and cardiovascular instability, blood was administered at the discretion of the anesthesia or intensive care teams. Coagulation factors and platelets were administered in response to bleeding, in the presence of coagulopathy or decreased platelet counts. The intraoperative salvage and homologous blood transfusion data are provided in Table 2.
Results of intraoperative salvage and homologous blood use.
| Mean | Minimum | Maximum | |
|---|---|---|---|
| Vol. Processed (ml) | 3400 | 680 | 7,609 |
| Vol. Surgical Field (ml) | 2669 | 280 | 6,000 |
| Vol. Concentrate (ml) | 616 | 126 | 1,800 |
| Units Salvaged (ml) | 1.55 | 0.5 | 4.3 |
| Total units salvaged | 18.5 | ||
| Total vol. salvaged (ml) | 32,027 |
| Blood use: | Patients | Percentage (%) |
|---|---|---|
| Operating room | 2 | 16.7 |
| ICU | 1 | 8.3 |
| Ward | 1 | 8.3 |
| Operating room+ICU | 3 | 25 |
| Operating room+ICU+Ward | 2 | 16.7 |
| Did not use blood | 3 | 25 |
| Used platelets | 4 | 33.3 |
| Used cryoprecipitate | 0 | 0 |
| Death | 0 | 0 |
Samples were collected according to the described protocol. The laboratory evaluation of salvaged blood was performed in two phases. In the first phase, prior to blood processing, the cardiotomy reservoir was gently shaken to homogenize the blood that had been aspirated from the patient. A volume between 100 and 200ml was pumped into the bowl, before the first sample (pre-sample) was collected. Using a 20-ml syringe, 18ml of blood was slowly aspirated, which were used to fill four tubes. The vacuum was used only with the citrate tube to generated the necessary proportion of blood and citrate (4.5ml and 0.5ml, respectively) to measure anti-Xa, which determines the amount of residual heparin. Two tubes with heparin were carefully filled with 4.0ml of blood each, after removing their lids to prevent hemolysis. One tube was used to determine the degree of hemolysis. The other heparin tube was used to determine the albumin and total protein concentrations. A fourth EDTA tube was filled with 4ml of blood for the determination of the hemoglobin (Hb) and hematocrit (Hct) levels and the red blood cell, platelet, and leukocyte counts.
In the second phase, samples (post-sample) were collected by removing the reinfusion bag at the end of the first full cycle of autologous blood salvage, without operator interference. The same collection protocol described above for the previous phase was followed. Immediately after collection, all samples were sent to the laboratory to ensure immediate processing.
Laboratory analysis of samplesAll cell counts (erythrocytes, leukocytes, platelets, hematocrit and hemoglobin concentration) were performed using an automated cell counter (Sysmex XT 2000i, Kobe, Japan). Total protein and albumin levels were measured using an automated colorimetric method and Vitros TP Slides (Vitros Chemistry Products), respectively, using a Vitros 5600 Integrated System (Ortho Clinical Diagnostics, Rochester, NY, USA). Anti-Xa levels were measured by the automated chromogenic method, using a HemosIL Liquid Anti-Xa kit with a Werfen ACL TOP 750 (Bedford, USA). The hemolysis index was determined using an SP 2000UV - Analyzer spectrophotometer.
Statistical analysisPatient demographics were obtained from patient records and charts. Laboratory data were obtained from the computerized system of the Hospital do Coração. Numerical data were analyzed by Fisher’s exact test, Student’s t test, the McNemar test and the non-parametric Wilcoxon test, as appropriate. P-values less than 0.05 were considered statistically significant.
ResultsThree patients (25%) did not receive any homologous blood units. The mean volume of the washed autologous red blood cell concentrate salvaged during surgery was 615ml/patient, ranging from 126 to 1800ml, with a standard deviation of 599.8ml. The total number of autologous blood units recovered from the washed red blood cell concentrate was 18.5, with a mean of 1.5 (range: 0.5 to 4.3) per patient. The hematocrit and hemoglobin levels were very similar between collection time points, 37.4% and 12.1g/dl in the pre- and 37.5% and 11.6g/dl in the post-operative period. A total of 45 homologous red blood cell (RBC) units were used. Three patients (25%) did not received any homologous blood units and eight patients (66.7%) received 18 homologous RBC units, averaging 2.25/patient. Only one patient (8.3%) received more than 4 units. The overall mean was 3.75/patient homologous RBC units.
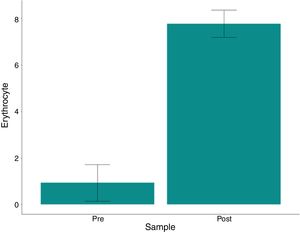
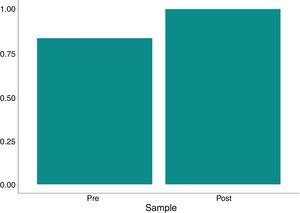
The variation in platelet and leukocyte concentrations between the pre- and post- processing samples was 8667 and 6,892, respectively. The p-values (paired t-test) were 0.592 and 0.01 for these same variables; thus, the difference was not statistically significant. The hematocrit and hemoglobin levels and red blood cell counts were significantly higher (p<0.001) in the post-processing samples compared to the pre-samples, with a mean variation of 54.78%, 19.81g/dL and 6.84×106/mm3, respectively, as shown in Table 3 and Figs. 1–3.
Hemoglobin and hematocrit levels, platelet, erythrocyte and leukocyte counts and hemolysis index.
| Hemoglobin | Minimum | Median | Mean | Maximum | Standard deviation | p-Value | p-Value (Wilcoxon) |
|---|---|---|---|---|---|---|---|
| Pre- | 0.8 | 2 | 2.73 | 8.5 | 2.18 | ||
| Post- | 19.9 | 22.7 | 22.54 | 24.9 | 1.59 | ||
| Variationa | 12.3 | 19.6 | 19.81 | 23.4 | 3.13 | <0.001 | <0.001 |
| Variation %b | 145 | 1,039 | 1,196 | 2,788 | 837 | <0.001 | |
| Hematocrit | |||||||
| Pre- | 2.5 | 6.55 | 8.62 | 26.3 | 6.74 | ||
| Post- | 57.2 | 63.5 | 63.49 | 67.5 | 2.95 | ||
| Variationa | 34.4 | 56.25 | 54.87 | 63.4 | 7.84 | <0.001 | <0.001 |
| Variation %b | 131 | 895 | 1,027 | 2,408 | 691 | <0.001 | |
| Platelets | |||||||
| Pre- | 7,000 | 26,000 | 24,778 | 42,000 | 11,935 | ||
| Post- | 6,000 | 19,000 | 33,444 | 97,000 | 32,004 | ||
| Variationa | −36,000 | 0 | 8667 | 84,000 | 39,532 | 0.52 | 0.834 |
| Variation %b | −86 | 0 | 106 | 646 | 248 | 0.23 | 0.44 |
| Erythrocytes | |||||||
| Pre- | 0.26 | 0.7 | 0.94 | 2.97 | 0.79 | ||
| Post- | 6.52 | 7.89 | 7.77 | 8.81 | 0.59 | ||
| Variationa | 4.33 | 7.02 | 6.84 | 8.03 | 0.99 | <0.001 | <0.001 |
| Variation %b | 146 | 1,055 | 1,238 | 2,938 | 859 | <0.001 | |
| Leukocytes | |||||||
| Pre- | 460 | 1,347 | 1,822 | 6,800 | 2,079 | ||
| Post- | 3,200 | 8,719 | 8,714 | 15,558 | 4,179 | ||
| Variationa | −3,600 | 7,439 | 6,892 | 13,970 | 5,630 | 0.01 | 0.02 |
| Variation %b | −53 | 592 | 971 | 2,369 | 963 | 0.02 | |
| Hemolysis index post | 0.16 | 0.45 | 0.44 | 0.8 | 0.19 | ||
p-value: Student’s t-test was used. Hypothesis: the mean variation (percentage) was equal to 0.
p-value (Wilcoxon): the nonparametric paired Wilcoxon test was used. Hypothesis: the median of the difference was equal to 0.
No significant correlation was observed between the protein and albumin concentrations in the pre- and post-processing samples (p=0.4780 by Fisher’s test and p=0.16 by the McNemar test).
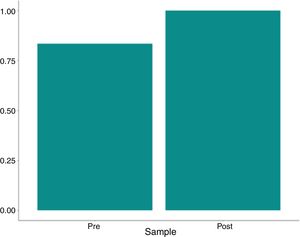
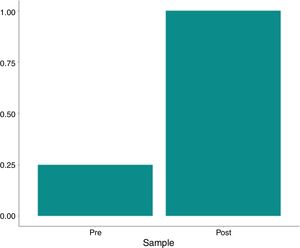
Residual heparin levels were less than or equal to 0.1IU/ml in all post-treatment analyses. The variation between the pre- and post-processing heparin concentration was significant (p=0.003 by the McNemar test and p<0.001 by Fisher's exact test), as shown in Table 4 and Figs. 4–6.
Residual heparin, total protein and albumin concentrations.
| Pre- | Post- | Total | p-Value | p-Valuea | ||
|---|---|---|---|---|---|---|
| Heparin | ||||||
| >0.1 | 9/12 (75%) | 0/12 (0%) | 9/24 (37.5%) | <0.001 | 0.003 | |
| ≤0.1 | 3/12 (25%) | 12/12 (100%) | 15/24 (62.5%) | |||
| Total Protein | ||||||
| ≥2 | 2/12 (16.7%) | 0/12 (0%) | 2/24 (8.3%) | 0.478 | 0.16 | |
| <2 | 10/12 (83.3%) | 12/12 (100%) | 22/24 (91.7%) | |||
| Albumin | ||||||
| ≥1 | 2/12 (16.7%) | 0/12 (0%) | 2/24 (8.3%) | 0.478 | 0.16 | |
| <1 | 10/12 (83.3%) | 12/12 (100%) | 22/24 (91.7%) | |||
p-Value: Comparison between the Pre and Post ratio. Fisher’s exact test.
Patients undergoing cardiac surgery, with or without extracorporeal circulation (ECC), are considered to be at increased risk for excessive bleeding, that is associated with complications. There is blood loss due to the involvement of several large vascular structures during the surgery, as well as hemostatic disorders associated with the use of ECC techniques, that frequently lead to the loss and destruction of red blood cells, platelets and coagulation factors during this procedure.17,18 These bleeding events can lead to an increased need for transfusion of allogeneic blood and hemostatic components, as well as surgical re-exploration.19–21 They are an important source of the total blood used for transfusion purposes, consuming approximately 10% to 15% of the national blood stock. Additional evidence suggests that this proportion is growing because of the increasing complexity of cardiac surgical procedures in adults and in congenital heart diseases.22,23 Real-world experiences, based on large samples of patients included in the database of the Society of Thoracic Cardiac Surgery in Cardiac Surgery in Adults, have shown that 50% of patients undergoing cardiac procedures receive allogeneic blood transfusion. Complex cardiac surgeries, such as reoperations, aortic surgeries, and implantation of ventricular assist devices, require blood transfusion with much greater frequency.24–26 However, it has been shown that allogeneic red blood cell transfusion is associated with short- and long-term complications, including infectious disease transmission and serious non-infectious transfusion risks,27 as well as increased mortality.28–30 This has led to the development of intraoperative blood salvage (cell saver technique), with the transfusion of autologous red blood cells as an important and effective blood conservation method, in order to reduce or avoid the need for transfusion of allogeneic red blood cells (and its associated complications).31 Compared with allogeneic blood, reinfused autologous red blood cells have more physiological concentrations of potassium, 2,3-diphosphoglycerate and adenosine triphosphate. These reinfused young and flexible erythrocytes maintain their biconcave shape, optimizing their viability and oxygen delivery.32,33 In a systematic review published in 2010 that included 75 trials (including 33 involving cardiac procedures and 6 involving vascular surgery), the absolute reduction in the use of allogeneic red blood cell transfusion was 21%.34 In another systematic review from 2015, in emergency abdominal or chest trauma (one trial, n=44), the reduction in the use of allogeneic red blood cells, associated with the use of the cell saver technique, was 4.7 units.35 These studies concluded that the use of the cell saver technique did not increase the risk for adverse events, including mortality and infection.35,36
In 2007, after an extensive review of the literature regarding perioperative blood transfusion and blood conservation in cardiac surgery,37 the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists reported that a minority of patients undergoing cardiac procedures (approximately 15–20%) consume more than 80% of transfused blood components during surgery. In other words, not all patients undergoing cardiac surgery share the same risks. The authors identified three broad factors that increase the risk for perioperative bleeding or blood transfusion: (1) advanced age, (2) decreased preoperative erythrocyte volume (anemia) and (3) emergency or complex surgeries (e.g., reoperations or aortic surgery). They suggested that patients at increased risk for bleeding are more likely to benefit from more aggressive blood conservation practices, particularly because patients at higher risk consume the majority of the blood resources.
Our present study data are consistent with the available literature, as only 3 patients (25%) consumed approximately 78% of the blood components used (2 of whom underwent reoperations of previous aortic surgeries, one of whom required a very long surgery and perfusion time (10h/415min); the third patient was old (73 years) and required surgical re-exploration). Most patients (75%) required allogeneic transfusion and 7 (58.4%) used a maximum of only 3 units throughout their hospital stay. We also observed that the use of the cell saver technique did not increase the risk for adverse events, including mortality and infection. The hemolysis index in the final washed product was below that recommended by current legislative guidelines (<0.8%, Table 3). The hematocrit and hemoglobin levels and the red blood cell counts were significantly higher (p<0.001) in the reinfusion bag, compared to the pre-sample results, with a mean value of 63.4, 22.5, and 7.7, respectively, indicating the excellent clinical performance and efficacy of the cellular salvage. This was highly consistent with the guidelines of the AABB,36 which indicate that the total hemoglobin concentration should be greater than 15g/dl. Avoiding homologous blood transfusion is not the only benefit of higher post-operative hemoglobin concentrations. Low hematocrit during and after surgery has been associated with a significant increase in mortality and severe morbidity, including the low cardiac output syndrome, intra-aortic pump use, and renal failure requiring dialysis.38
Washed autologous red blood cell concentrates containing significant amounts of heparin could cause post-extracorporeal circulation coagulopathy. The chromogenic anti-A assay is the current gold standard technique for measuring unfractionated heparin in citrated human plasma, based on the inactivation of factor Xa, in the presence of a chromogenic synthetic substrate. In this assay, excess antithrombin III and a known amount of factor Xa are added to the sample. The heparin-antithrombin III complexes that form neutralize a portion of the available factor Xa. The non-bound factor Xa releases the paranitroaniline chromogenic substrate, which is measured as the inverse of the amount of heparin present. In the definition of quality indicators for intraoperative blood salvage, the AABB guidelines state that the residual heparin level in the final product should be <0.5 anti-Xa lU/ml. Our data showed that residual heparin levels were ≤0.1IU.ml in all post-processing analyses (p<0.003 by the McNemar test and p<0.001 by Fisher's exact test).
In conclusion, the use of the XTRA Autotransfusion System-based cell saver technique in cardiac surgeries was safe and effective for blood conservation, via the transfusion of washed autologous red blood cells. The reduced residual heparin (≤0.1IU/ml) values in the processed blood are noteworthy. These results demonstrate that residual heparin concentrations in the final product are clinically insignificant and are unlikely to increase the risk of intra- and post-operative hemorrhage.
Conflicts of interestThe authors declare no conflicts of interest.