Anaplastic multiple myeloma is a rare morphological variant of multiple myeloma that shows an overly aggressive clinical course. It is characterized by the presence of pleomorphic and immature plasma cells, sometimes resembling megakaryocytes or even osteoclasts.1 It is more common in young patients and usually has a predisposition to the involvement of extramedullary sites.2 Cytogenetic abnormalities of poor prognosis such as 1q21 amplification, 17p deletion (p53), and chromosome 13 anomalies are common.3 This morphological variant is usually characterized as a chemo refractory disease, with an estimated mean survival of 3 to 5 months.4
Within the clinical spectrum of plasma cell dyscrasias, plasma cell leukemia (PCL) is, along with anaplastic multiple myeloma, at the extreme of greater clinical aggressiveness. PCL is a rare form of plasma cell dyscrasia, but its incidence is likely to be underestimated due to its stringent diagnostic criteria.5 PCL is in a process of change of its defining criteria as recent studies suggest that the presence of ≥ 5% circulating abnormal plasma cells have a similar poor prognostic impact as patients presenting with classic diagnostic parameter for PCL (>20% abnormal circulating plasma cell). Therefore, patients with such characteristics may be considered as having PCL and can be treated as such.6
In this manuscript, we report clinical and cytogenetic findings in a case of anaplastic multiple myeloma with abnormal circulating plasma cells suggestive of primary PCL.
Case reportA Bolivian male with no previous comorbidities presents with a 6-month history of lower back and rib cage pain associated with 20 kg weight loss and significant loss of functionality. There was no history of fever, lymphadenomegaly, or other symptoms. Physical assessment reveals rib cage deformity and muscle atrophy, with no other significant findings.
Complete blood cell count shows a hemoglobin count of 6.6 g/dl (MCV 93 fl; RDW 16,7%), white blood cell (WBC) of 9660/mm3; (Neutrophils: 7000/mm3; Lymphocytes: 1200/mm3; Monocytes: 1200/mm3), Platelet count of 239,000/mm3 and reticulocyte index of 1.15%.
Biochemical evaluation shows creatinine levels of 4.5 mg/dL (eGFR CKD-EPI 13ml/min/1.73 m2), potassium of 4.6mEq/L, ionized calcium of 2.07 mmol/L (1.16-1.32 mmol/L), phosphorus of 6.1mg/dL, uric acid 10 mg/dL (2.4-6 mg/dL) and albumin of 4.5 g/dL. Total bilirubin, ALT, and AST are within normal limits, while alkaline phosphatase is mildly elevated at 194U/L and gamma-glutamyl transferase is elevated at 393U/L (9-36U/L). Lactate dehydrogenase is within the normal limit at 267U/L.
A further diagnostic evaluation takes place due to the hypothesis of a plasma cell disorder. Serum protein electrophoresis shows a free kappa chain M-protein of 0.1 g/dL, while urinary evaluation shows a Kappa M-protein of 1,120 mg/24 h output. Kappa light chain measure is of 4,985 mg/L, while the Free light chain (FLC) ratio is 554. Levels of B2-microglobulin are at 33 mg/L.
Whole body CT scan shows multiple lytic bone lesions distributed diffusely, multiple pathological vertebral fractures, without extra-osseous tumor component.
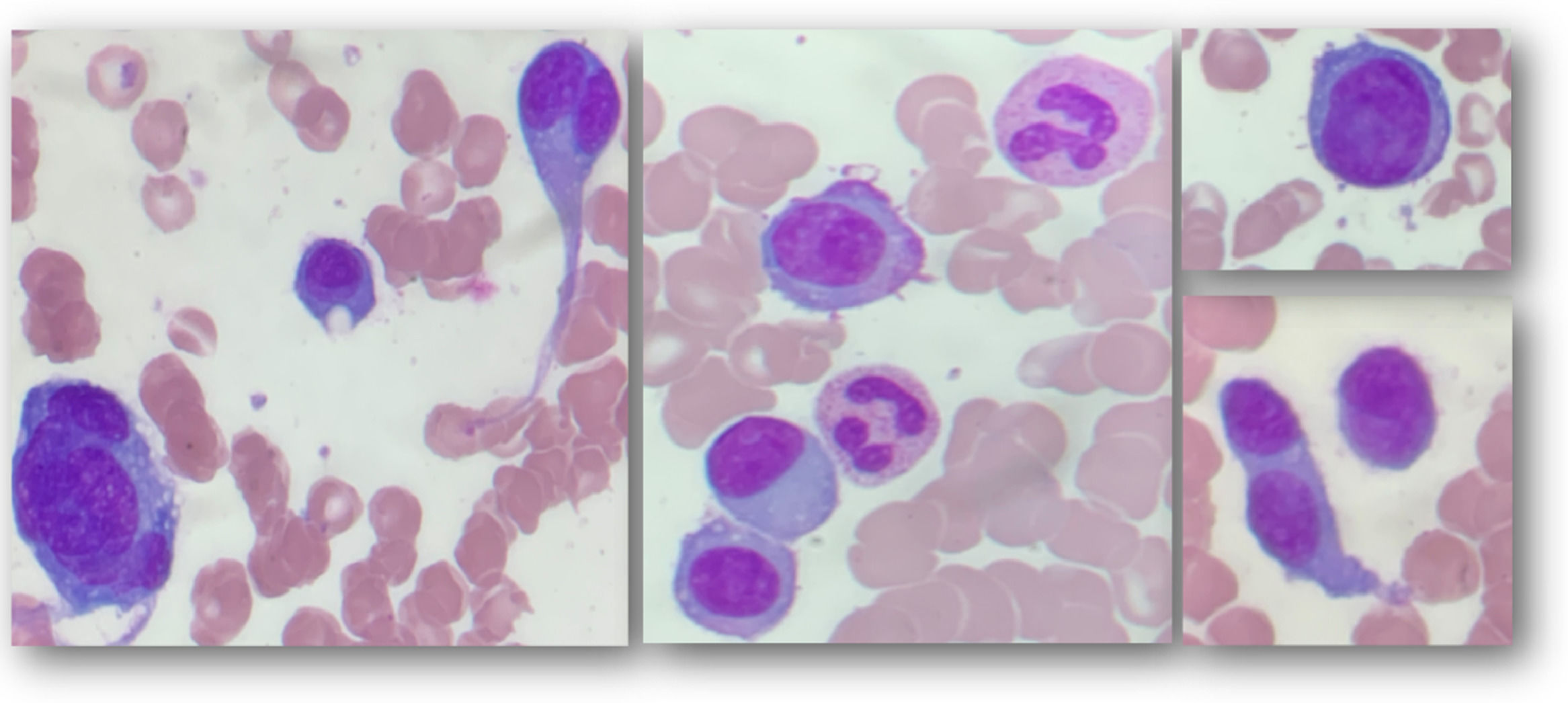
Bone marrow aspirate shows 46.4% pleomorphic plasma cells, usually of large size, some presenting convoluted nuclei; some binucleated, others multinucleated, with marked nucleolus (Figure 1); bone marrow biopsy is hypercellular with plasma cell infiltration (80% of nucleated cells) and increased reticulogenesis - MF2. Immunophenotyping by flow cytometry shows positivity in 34.49% of analyzed cells for CD38, CD138, CD117, kappa, and negativity for CD45, CD20, CD56, lambda.
Due to differential WBC showing repeated monocytosis and the distinctive pleomorphic plasma cell characterization seen in bone marrow aspirate, a peripheral blood smear is evaluated, revealing a 15% of leukocytes with morphology and immunophenotype similar to those seen in the bone marrow.
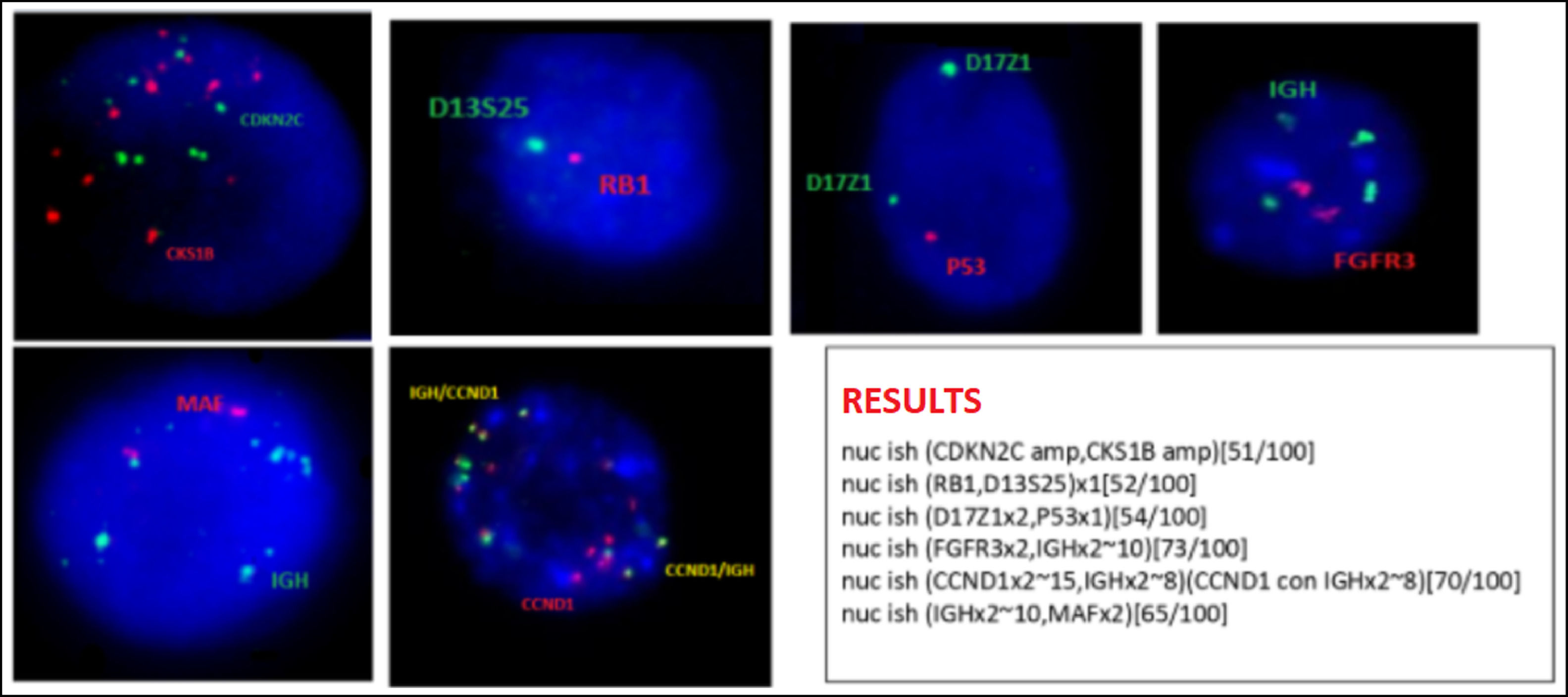
Bone marrow karyotype is diploid with no clonal abnormalities. Interphasic FISH in CD138-positive cells shows signals suggestive of amplification of the CCND1-IGH gene fusion in 70% of the analyzed nuclei; amplification of CKS1B and CDKN2C (chromosome 1), deletion of the RB1 gene and D13S25 region (chromosome 13q) and deletion of the P53 gene (chromosome 17p) in 50% of the nuclei (Figure 2).
Multiple Myeloma interphasic FISH panel - CD138+ cells (according to Kishimoto, 20167) A. many red (CDKN2A) and green (CKS1B) signals, compatible with amplification CKS1B and CDKN2A genes; B. one red (RB1) and one green (D13S25) signal, compatible with deletion/monosomy 13; C. two green (centromere 7) and 1 red signal (P53) compatible with P53 deletion; D. 2 red (FGFR3) and many green (IGH) signals; E 2 red (MAF) and many red (IGH) signals; F: many red (CCND1) and green (IGH) fusion signals compatible with CCND1-IGH fusion amplification. Cut-off levels according to European Myeloma Network (EMN) guideline published in 2012: 10% for fusion or breakapart probes [t(4;14); t(11;14); t(14;16)], and 20% for numerical abnormalities [gain or deletion of CK1SB, CDKN2C (chromosome 1), TP53 (17p) and RB1 (13q)].8
With an established diagnosis of free kappa anaplastic multiple myeloma/ primary PCL, therapy is initiated with Bortezomib IV 1.5 mg/m2/weekly; oral Cyclophosphamide 300 mg/m2/weekly; oral Dexamethasone 40 mg/weekly (intensive chemotherapy treatment not started due to poor Performance Status – ECOG score of 3). Evaluation after the sixth cycle of VCd shows no mensurable serum M-protein at electrophoresis and immunofixation. Urinary immunofixation persists, but without a measurable monoclonal peak. There is a reduction in FLC ratio to 43.45 (free kappa 302; free lambda 6.95), in addition to improvement in hemoglobin levels (9.1 g/dL) and in renal function (Creatinine 0.95 mg/dL; eGFR CKD-EPI 85.1 ml/min/1.73 m2). Despite these improvements, patient persists with ECOG of 3, currently not being a candidate for treatment intensification.
DiscussionWe present a case of a patient diagnosed with anaplastic multiple myeloma / primary PCL, correlating clinical findings of aggressive disease with anaplastic morphology of the plasma cells and adverse cytogenetic findings.
PCL is an aggressive rare plasma cell dyscrasia that, when compared to multiple myeloma, usually presents in younger patients, with frequent extramedullary involvement and worst patient performance status at diagnosis, indicating a more aggressive clinical course and therefore requiring intensified therapy to achieve better response rates.9
M-protein in PCL is typically IgG, although light chain is seen in up to 40% of cases. The disease typically has an initial presentation suggesting a more advanced clinical stage, with frequent cytopenias due to bone marrow infiltration, elevated lactate dehydrogenase, and elevated B2-microglobulin.10
Cytogenetical findings in PCL also usually differ from other plasma cell dyscrasias. In the former, there are more frequent high-risk cytogenetic abnormalities, like del 17p (56%), 1q gain (51%), and hypodiploidy (70%). The presence of t(11;14), a finding found in up to 15% of patients with multiple myeloma and an indicator of clinical response to the use of Venetoclax, is often found in PCL (cytogenetic abnormality seen in up to 70% of cases).11 Metaphase chromosome analysis in plasma cell dyscrasias can be challenging due to the low mitotic index of plasma cells, which makes conventional cytogenetic difficult. Therefore, chromosomal analysis of clonal plasma cell in multiple myeloma/ PCL is a difficult challenge. In this scenario, FISH has been proven useful as it increased the detection rate of genetic abnormalities, especially if it is performed in selected plasm cells.7,12
The patient in question had 15% of plasma cells in peripheral blood. Although this finding does not characterize PCL based on classic diagnostic criteria,13 the diagnostic presumption was made due to clinical characteristics indicative of aggressive disease and loss of CD56 expression in the cells of interest (a finding associated with primary PCL).14
The cytogenetic findings in this case corroborate the aggressive profile of clinical manifestations presented by the patient, being an important prognostic indicator. Based on the Mayo Clinic's mSMART 3.0 risk stratification,15 this patient presents adverse cytogenetic findings, such as 17p (p53) deletion and 1q21 amplification, characterizing it as a double hit multiple myeloma. The patient also presents deletion on chromosome 13q, a finding associated with worse prognosis particularly if it is expressed in a high proportion of malignant cells and associated to higher cell proliferation rate.16
Fluorescence in situ hybridization (FISH) assessment shows amplification of the CCND1-IGH gene fusion in 70% of the plasma cells. Although little is known about the molecular basis of pPCL, t(11;14) appears to be important in the pathogenesis of the disease.17 Overexpression of cyclin D1 caused by the CCND1-IGH gene fusion leads to an increase in the transition from G1 to S phase of the cell cycle, resulting in higher rates of neoplastic cell proliferation and consequently a more aggressive disease. It is relevant to report that the presence of CCND1-IGH fusion gene can occur even without the finding of t(11;14) in a conventional karyotype.18 Therefore, it is important to study this gene fusion in patients with pPCL who do not have t(11;14) detected in the karyotype.
Although t(11;14) - CCND1-IGH gene fusion is frequently found in plasma cell dyscrasias, amplification of this gene fusion is a very rare finding and is particularly described in pPCL.18 This finding could have an important role in the pathogenesis of this disease, maybe being responsible for an even more aggressive clinical course than that of a unique CCND1-IGH gene fusion.
Dysregulation of the apoptotic pathways depicts one of several mechanisms for tumor cell survival. Overexpression of the anti-apoptotic protein BCL-2 is present in multiple myeloma and represents a potential therapeutic target.19 Recent evidence suggests that the use of Venetoclax, a drug that inhibits the anti-apoptotic protein BCL-2, has clinical benefit in multiple myeloma, particularly in those with t(11;14).20 The use of bortezomib and dexamethasone associated with either Venetoclax or placebo in patients with relapsed or refractory multiple myeloma after 3 lines of treatment was evaluated in a phase III study.21 The group that received Venetoclax had better PFS rates (22.4 vs. 11.5 months, p = 0.01). Particularly, the subgroup of patients with t(11;14) showed better rates of complete or very good partial response in the arm that received Venetoclax, reinforcing the molecular rationale for using the drug in this particular situation. Studies evaluating the use of Venetoclax in patients with PCL presenting t(11;14) are necessary, but evidence may be extrapolated in an individual case scenario.
ConclusionThis case shows two interesting aspects of plasma cell dyscrasia: the involvement of peripheral blood by anaplastic plasma cells and the amplification of the CCND1-IGH gene fusion. Recent studies have shown the potential effectiveness of BCL2 inhibitors in multiple myeloma with t(11;14). Newer studies including patients with PCL are needed to confirm similar outcomes in this clinical scenario.



![Multiple Myeloma interphasic FISH panel - CD138+ cells (according to Kishimoto, 20167) A. many red (CDKN2A) and green (CKS1B) signals, compatible with amplification CKS1B and CDKN2A genes; B. one red (RB1) and one green (D13S25) signal, compatible with deletion/monosomy 13; C. two green (centromere 7) and 1 red signal (P53) compatible with P53 deletion; D. 2 red (FGFR3) and many green (IGH) signals; E 2 red (MAF) and many red (IGH) signals; F: many red (CCND1) and green (IGH) fusion signals compatible with CCND1-IGH fusion amplification. Cut-off levels according to European Myeloma Network (EMN) guideline published in 2012: 10% for fusion or breakapart probes [t(4;14); t(11;14); t(14;16)], and 20% for numerical abnormalities [gain or deletion of CK1SB, CDKN2C (chromosome 1), TP53 (17p) and RB1 (13q)].8 Multiple Myeloma interphasic FISH panel - CD138+ cells (according to Kishimoto, 20167) A. many red (CDKN2A) and green (CKS1B) signals, compatible with amplification CKS1B and CDKN2A genes; B. one red (RB1) and one green (D13S25) signal, compatible with deletion/monosomy 13; C. two green (centromere 7) and 1 red signal (P53) compatible with P53 deletion; D. 2 red (FGFR3) and many green (IGH) signals; E 2 red (MAF) and many red (IGH) signals; F: many red (CCND1) and green (IGH) fusion signals compatible with CCND1-IGH fusion amplification. Cut-off levels according to European Myeloma Network (EMN) guideline published in 2012: 10% for fusion or breakapart probes [t(4;14); t(11;14); t(14;16)], and 20% for numerical abnormalities [gain or deletion of CK1SB, CDKN2C (chromosome 1), TP53 (17p) and RB1 (13q)].8](https://static.elsevier.es/multimedia/25311379/0000004500000004/v1_202310231039/S2531137921013109/v1_202310231039/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w93OM6WmS6o9DeZl+SVh74uo=)



