Allogeneic hematopoietic stem cell transplantation (allo-SCT) is a well-established treatment modality for myelofibrosis (MF) and the only potentially curative treatment. However, adequate engraftment, relapse and transplant related mortality are big challenges in transplantation for this disease.
ObjectivesPrimary objective is to evaluate overall and disease-free survival of allogeneic HSCT for myelofibrosis in Brazil. Secondary objective is identifying risk factors for survival and relapse.
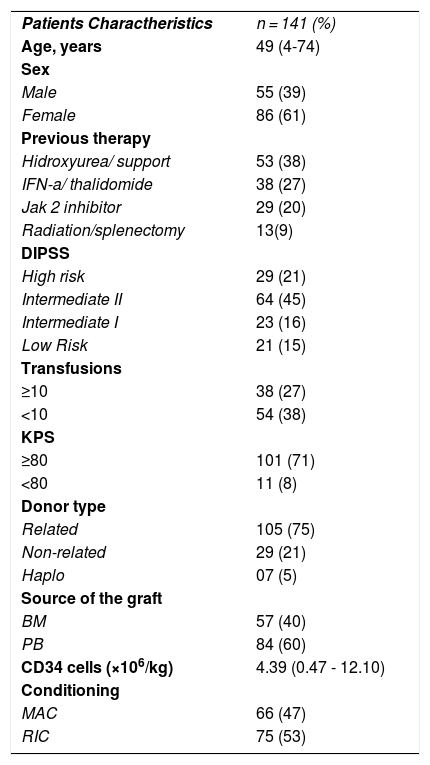
Patients and methodsWe analyzed retrospectively from charts and database data from 141 patients with the diagnosis of MF who received allo-SCT at six centers in Brazil from 1997 to 2020. Patients and Transplant characteristics are disposed on Table 1. Statistical analysis was performed using EZR software. Bivariate analysis and estimation of OS and DFS were performed using Log Rank and Kaplan Meier method. A landmark analysis of survival was performed for acute (100 days) and chronic (180 days) GVHD. Cox Survival Model was used for multivariate analysis of survival and Multivariable Fine-Gray competing risks regression model was used or relapse/rejection at 2 years and non-relapse mortality at one year. Characteristics of patients are showed in table 1.
ResultsOverall Survival (OS) for the whole cohort was 61% at one year, 56% at 2 years and 46% at 5 years. The transplant related mortality at 100 days and at one year was 14 and 26%, respectively. One- and two-year cumulative incidence of relapse or rejection was 10 % and 15% respectively. Cumulative incidence of non-relapse mortality (competitive risk) at one and two years was 33% and 37% respectively. At multivariate analysis by Cox Model, CD 34 > 5 x 106 HR 0.4405 (0.2396- 0.8101; p = 0.008) was associated to better survival, while KPS < 80% HR 2.3010 (1.01-5.19; p = 0.044) and DIPSS intermediate 2 or high-risk HR 5.7490 (2.33-14.18; p = 0.0001) were significantly associated with inferior survival. At Multivariable Fine-Gray competing risks regression model for relapse/rejection, previous therapy with ruxolitinib was identified as a risk factor HR 3.245(1.427- 7.379; p = 0.005), but there were only ten events in this group. At Multivariable Fine-Gray competing risks regression model for non-relapse mortality, KPS < 80% HR 3.2380(1.3030-8.046; p = 0.011) and DIPSS Intermediate 2-High risk were identified as independent risk factors -HR 8.8760 (1.9280 -40.870; p = 0.00510). At landmark analysis development of acute GVHD stage 3-4 was a significant risk factor for survival (0.00194).
Conclusions1) DIPSS score was validated in this Brazilian cohort as a tool to estimate survival and non-relapse mortality after transplant, in agreement with international experience; 2) CD34 dose < 5 x 106 and Karnofsky score < 80% were significant risk factors for survival; 3) Karnofsky score < 80% and Intermediate-2 or High risk DIPSS were also significant risk factors for non-relapse mortality.