Invasive fungal diseases represent important causes of morbidity and mortality among pediatric oncohematological patients. Acute invasive fungal rhinosinusitis is a rare and aggressive disease that occurs mainly in immunocompromised patients. The mortality rate is high and therefore, accurate and early diagnosis is essential.
ObjectivesThe aim of this study was to describe the frequency of acute invasive fungal rhinosinusitis among pediatric oncohematological patients and characterize them with confirmed diagnoses.
MethodsThis was a retrospective study that analyzed the medical records of pediatric patients diagnosed with oncohematological diseases and suspected fungal infections, who were included after obtaining informed consent, from January to December 2017, in the pediatric unit of a tertiary university hospital. Data collected from medical record analysis included the following: underlying diagnosis, absolute neutrophil count, clinical presentation, culture and biopsy results, surgical procedures performed, survival and mortality.
ResultsA total of 27 patients were evaluated, with three suspected cases of acute invasive fungal rhinosinusitis. Histopathological and microbiological analyses confirmed two cases. In both cases, the pathogen isolated in the culture was Fusarium sp. The two confirmed cases were female, aged 12 and 14 years, both with an absolute neutrophil count of 10cells/μL. The underlying disease of the first patient was acute myeloid leukemia (subtype M5), whereas the second patient presented idiopathic bone marrow aplasia.
ConclusionBoth confirmed cases of acute invasive fungal rhinosinusitis presented with constitutional symptoms and signs of nasal and sinusital inflammation. This demonstrates the importance of fever as a symptom in immunocompromised patients and it should prompt otorhinolaryngological investigation.
Invasive fungal diseases (IFDs) represent important causes of morbidity and mortality among pediatric oncohematological patients. Early diagnosis and treatment of an IFD is associated with a better outcome and this requires the use of fast and precise methods that can support clinicians in its management.1
Fungal rhinosinusitis (FRS) can be classified as invasive or noninvasive, based on the histopathological evidence of tissue invasion by the fungus, which results in necrosis and local tissue destruction. This invasive disease is subdivided into acute invasive FRS (AIFRS), granulomatous invasive FRS and chronic invasive FRS.2–4
AIFRS is a rare and aggressive disease that occurs mainly in immunocompromised patients, particularly in those with neutropenia, with oncohematological diseases representing a major predisposing factor.4,5 Infection is characterized by fungal invasion of the nasal or paranasal cavity, which may affect adjacent organs, such as the orbits and intracranial structures.6 Numerous fungi can cause invasive infection, however, the most commonly identified fungi in AIFRS are Aspergillus and Zygomycetes (Rhizopus, Mucor, Rhizomucor).2,7–10
The diagnosis is challenging and fever is often the only presenting symptom. Other signs and symptoms may be very slight due to the reduced local inflammatory response capacity, which is a consequence of neutropenia. The complications include delayed chemotherapy treatment that may result in cancer recurrence, bone erosion, orbital invasion, brain abscess, meningitis, hematogenous spread and death. The mortality rate is high, ranging from 20 to 80% and therefore, accurate and early diagnosis is essential.5,7,11–13
The aim of this study was to assess the frequency of AIFRS among pediatric oncohematological patients evaluated by the otorhinolaryngology service and determine the characteristics of patients with confirmed diagnoses.
MethodsThe study was approved by the Institutional Research Ethics Committee (number 1.425.036) and participants were included after proxy informed consent was obtained from the children’s guardians.
Patients diagnosed with malignant neoplasia or aplastic anemia from January to December 2017 were included.
Inclusion criteria were patients aged less than 18 years, hospitalized for the treatment of oncohematological diseases and who exhibited indications for otorhinolaryngological evaluation and possible infection, as well as neutropenia (a neutrophil count of <500/μL or <1,000/μL, with a tendency to undergo further decrease), fever (axillary temperature ≥37,8°C) and/or nasosinusal symptoms.
Medical record analysis collected the following data: underlying diagnosis, absolute neutrophil count, clinical presentation, culture and biopsy results, surgical procedure performed and clinical outcome.
The medical records were reviewed and the data were collected by a multidisciplinary team responsible for the routine care of oncohematological patients. In this period, the team met regularly to discuss and standardize data registration in a previously prepared form. All study members were trained in data collection and regular meetings were held to discuss methodology and findings.
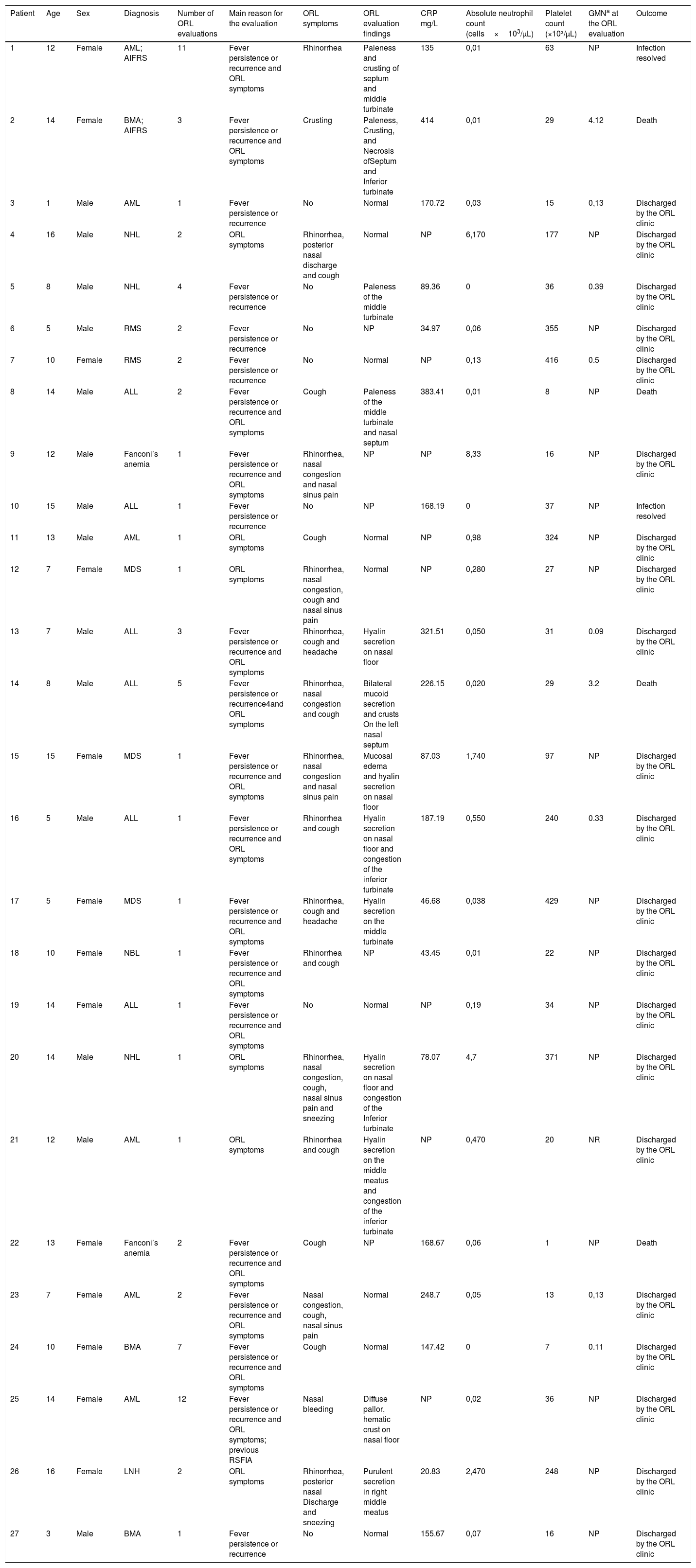
ResultsIn total, 27 patients were evaluated and among three suspected cases of AIFRS, two cases (7.41% 95% CI: 0.9–24.3) were confirmed with histopathological and microbiological evidence of fungal infection (Table 1). The unconfirmed patient died before undergoing the surgical approach.
Clinical/laboratory data of patients in a retrospective cohort of immunocompromised patients at a cancer reference center.
| Patient | Age | Sex | Diagnosis | Number of ORL evaluations | Main reason for the evaluation | ORL symptoms | ORL evaluation findings | CRP mg/L | Absolute neutrophil count (cells×103/μL) | Platelet count (×10³/μL) | GMNa at the ORL evaluation | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 | Female | AML; AIFRS | 11 | Fever persistence or recurrence and ORL symptoms | Rhinorrhea | Paleness and crusting of septum and middle turbinate | 135 | 0,01 | 63 | NP | Infection resolved |
| 2 | 14 | Female | BMA; AIFRS | 3 | Fever persistence or recurrence and ORL symptoms | Crusting | Paleness, Crusting, and Necrosis ofSeptum and Inferior turbinate | 414 | 0,01 | 29 | 4.12 | Death |
| 3 | 1 | Male | AML | 1 | Fever persistence or recurrence | No | Normal | 170.72 | 0,03 | 15 | 0,13 | Discharged by the ORL clinic |
| 4 | 16 | Male | NHL | 2 | ORL symptoms | Rhinorrhea, posterior nasal discharge and cough | Normal | NP | 6,170 | 177 | NP | Discharged by the ORL clinic |
| 5 | 8 | Male | NHL | 4 | Fever persistence or recurrence | No | Paleness of the middle turbinate | 89.36 | 0 | 36 | 0.39 | Discharged by the ORL clinic |
| 6 | 5 | Male | RMS | 2 | Fever persistence or recurrence | No | NP | 34.97 | 0,06 | 355 | NP | Discharged by the ORL clinic |
| 7 | 10 | Female | RMS | 2 | Fever persistence or recurrence | No | Normal | NP | 0,13 | 416 | 0.5 | Discharged by the ORL clinic |
| 8 | 14 | Male | ALL | 2 | Fever persistence or recurrence and ORL symptoms | Cough | Paleness of the middle turbinate and nasal septum | 383.41 | 0,01 | 8 | NP | Death |
| 9 | 12 | Male | Fanconi’s anemia | 1 | Fever persistence or recurrence and ORL symptoms | Rhinorrhea, nasal congestion and nasal sinus pain | NP | NP | 8,33 | 16 | NP | Discharged by the ORL clinic |
| 10 | 15 | Male | ALL | 1 | Fever persistence or recurrence | No | NP | 168.19 | 0 | 37 | NP | Infection resolved |
| 11 | 13 | Male | AML | 1 | ORL symptoms | Cough | Normal | NP | 0,98 | 324 | NP | Discharged by the ORL clinic |
| 12 | 7 | Female | MDS | 1 | ORL symptoms | Rhinorrhea, nasal congestion, cough and nasal sinus pain | Normal | NP | 0,280 | 27 | NP | Discharged by the ORL clinic |
| 13 | 7 | Male | ALL | 3 | Fever persistence or recurrence and ORL symptoms | Rhinorrhea, cough and headache | Hyalin secretion on nasal floor | 321.51 | 0,050 | 31 | 0.09 | Discharged by the ORL clinic |
| 14 | 8 | Male | ALL | 5 | Fever persistence or recurrence4and ORL symptoms | Rhinorrhea, nasal congestion and cough | Bilateral mucoid secretion and crusts On the left nasal septum | 226.15 | 0,020 | 29 | 3.2 | Death |
| 15 | 15 | Female | MDS | 1 | Fever persistence or recurrence and ORL symptoms | Rhinorrhea, nasal congestion and nasal sinus pain | Mucosal edema and hyalin secretion on nasal floor | 87.03 | 1,740 | 97 | NP | Discharged by the ORL clinic |
| 16 | 5 | Male | ALL | 1 | Fever persistence or recurrence and ORL symptoms | Rhinorrhea and cough | Hyalin secretion on nasal floor and congestion of the inferior turbinate | 187.19 | 0,550 | 240 | 0.33 | Discharged by the ORL clinic |
| 17 | 5 | Female | MDS | 1 | Fever persistence or recurrence and ORL symptoms | Rhinorrhea, cough and headache | Hyalin secretion on the middle turbinate | 46.68 | 0,038 | 429 | NP | Discharged by the ORL clinic |
| 18 | 10 | Female | NBL | 1 | Fever persistence or recurrence and ORL symptoms | Rhinorrhea and cough | NP | 43.45 | 0,01 | 22 | NP | Discharged by the ORL clinic |
| 19 | 14 | Female | ALL | 1 | Fever persistence or recurrence and ORL symptoms | No | Normal | NP | 0,19 | 34 | NP | Discharged by the ORL clinic |
| 20 | 14 | Male | NHL | 1 | ORL symptoms | Rhinorrhea, nasal congestion, cough, nasal sinus pain and sneezing | Hyalin secretion on nasal floor and congestion of the Inferior turbinate | 78.07 | 4,7 | 371 | NP | Discharged by the ORL clinic |
| 21 | 12 | Male | AML | 1 | ORL symptoms | Rhinorrhea and cough | Hyalin secretion on the middle meatus and congestion of the inferior turbinate | NP | 0,470 | 20 | NR | Discharged by the ORL clinic |
| 22 | 13 | Female | Fanconi’s anemia | 2 | Fever persistence or recurrence and ORL symptoms | Cough | NP | 168.67 | 0,06 | 1 | NP | Death |
| 23 | 7 | Female | AML | 2 | Fever persistence or recurrence and ORL symptoms | Nasal congestion, cough, nasal sinus pain | Normal | 248.7 | 0,05 | 13 | 0,13 | Discharged by the ORL clinic |
| 24 | 10 | Female | BMA | 7 | Fever persistence or recurrence and ORL symptoms | Cough | Normal | 147.42 | 0 | 7 | 0.11 | Discharged by the ORL clinic |
| 25 | 14 | Female | AML | 12 | Fever persistence or recurrence and ORL symptoms; previous RSFIA | Nasal bleeding | Diffuse pallor, hematic crust on nasal floor | NP | 0,02 | 36 | NP | Discharged by the ORL clinic |
| 26 | 16 | Female | LNH | 2 | ORL symptoms | Rhinorrhea, posterior nasal Discharge and sneezing | Purulent secretion in right middle meatus | 20.83 | 2,470 | 248 | NP | Discharged by the ORL clinic |
| 27 | 3 | Male | BMA | 1 | Fever persistence or recurrence | No | Normal | 155.67 | 0,07 | 16 | NP | Discharged by the ORL clinic |
AML: acute myeloid leukemia; AIFRS: acute invasive fungal rhinosinusitis; ORL: otorhinolaryngology; BMA: bone marrow aplasia; NHL: non-Hodgkin’ lymphoma; RMS: rhabdomyosarcoma; ALL: acute lymphoblastic leukemia; MDS: myelodysplastic syndrome; NBL: neuroblastoma; CRP: C-reactive protein, PLAT: platelets, NP: not performed.
The two confirmed cases of AIFRS were female, aged 12 and 14 years, and both had an absolute neutrophil count of 10cells/μL. The first patient (P1) had acute myeloid leukemia (subtype M5) as an underlying disease and was in the induction phase of the treatment, with an interval of 15 days between the beginning of chemotherapy and the first otorhinolaryngological evaluation. The second patient (P2) was diagnosed with severe aplastic anemia.
Fever, hyaline rhinorrhea and nasal crusting were the symptoms observed in the two patients with confirmed diagnoses. Patient P1 presented with fever recurrence after 12 days of being afebrile under meropenem treatment for 14 days. Patient P2 presented with persistent fever for 23 days, despite the use of meropenem, polymyxin, liposomal amphotericin, linezolid and micafungin (Table 1).
In both cases, the sites of nasal involvement were the anterior septum, associated with a lesion in the left inferior turbinate or right middle turbinate, with direct examination revealing adhered crusts and pale mucosa. However, patient P2 had evolved to necrosis at the time of surgery. Only patient P1 underwent sinus computed tomography, which revealed nonspecific findings of mucosal thickening of the maxillary, frontal and sphenoid sinuses.
Both patients received treatment with a systemic antifungal (liposomal amphotericin and liposomal amphotericin associated with micafungin) and surgical debridement. No granulocyte colony-stimulating factor was used. The surgical samples removed were sent for histopathological examination and culture. In both cases, the pathogen isolated in the cultures was Fusarium sp. Histopathological evaluation revealed respiratory mucosa with areas of necrosis and the presence of numerous filamentous fungi with hyaline, acute-branching septate hyphae.
Weekly postoperative follow-up was performed, with nasosinusal endoscopy in P1, until neutrophil recovery. Recurrent infection or post-operative complications, such as pain, vomiting, bleeding, infection, abscess, or synechiae were not observed. Patient P2 died 6 days after surgery with septic shock and multiple-organ failure.
DiscussionAIFRS is a severe opportunistic infection with high morbidity and mortality that usually occurs in immunosuppressed patients. Risk factors include hematologic malignancies and aplastic anemia, which lead to the reduction in, and dysfunction of, neutrophils.2–5 As in adults, the most important risk factor in pediatric patients is hematologic neoplasia, probably due to the long periods of intense neutropenia that these patients often experience during their treatment.6,7
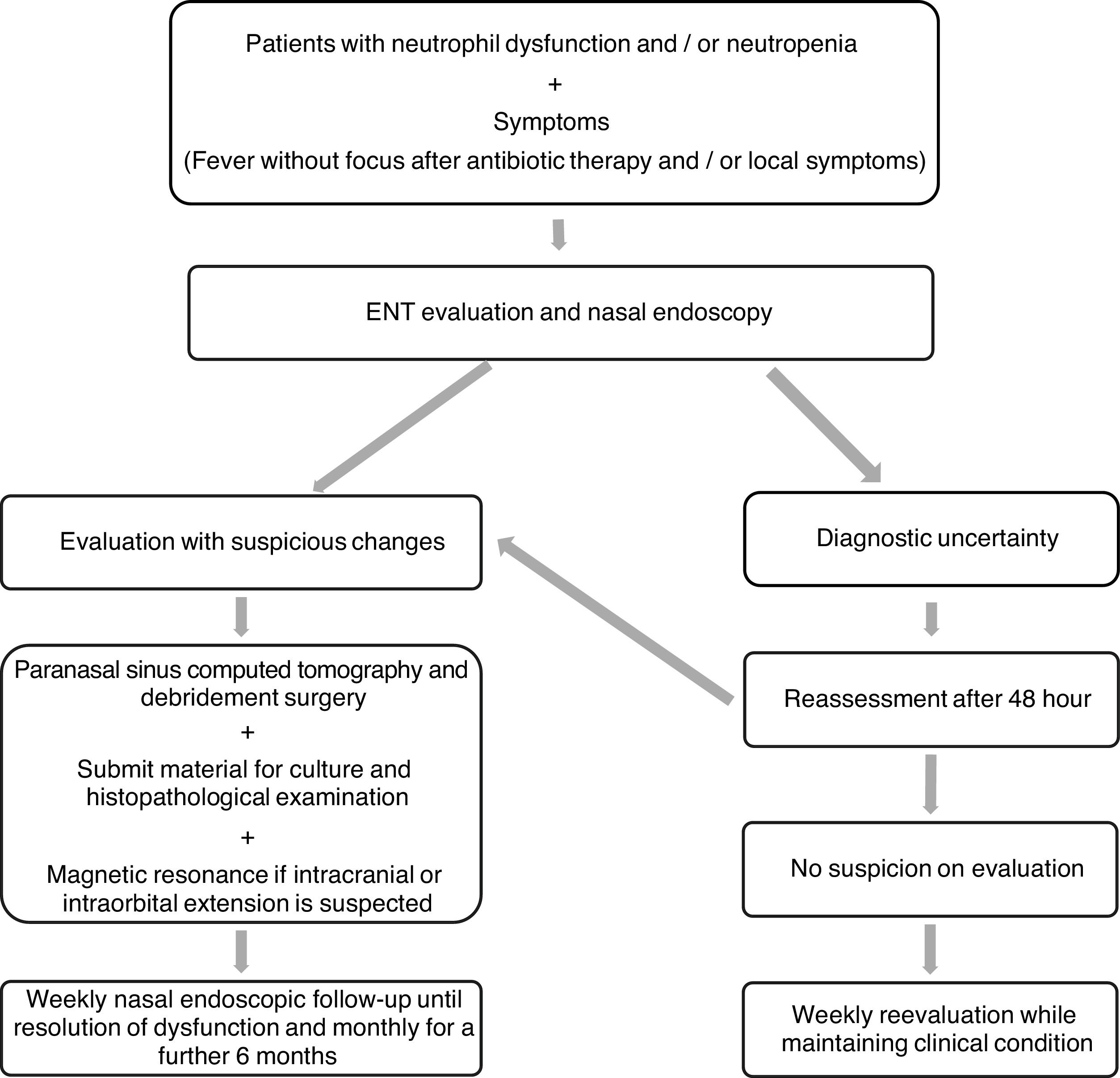
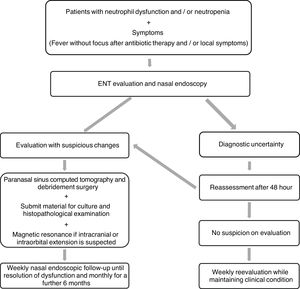
Upper airway fungal infection should be investigated whenever a neutropenic patient continues to exhibit persistent or recurrent fever after 4–7 days of broad-spectrum antibiotic therapy or exhibits signs and symptoms suggestive of airway infection, such as persistent cough, rhinorrhea and nasal obstruction.14
The most common initial symptoms of AIFRS are fever, nasal discharge, facial pain, rhinorrhea and nasal congestion.11,15,16 Similarly, we observed nonspecific early symptoms including fever, hyaline rhinorrhea and nasal crust formation. Visual and neurological symptoms, such as ophthalmoplegia, proptosis, orbital cellulitis, visual loss, changes in mental status and palate erosion, are signs of an extensive disease and indicate orbital or intracranial invasion.17–19
The presence of such symptoms in immunocompromised patients is the condition requiring mandatory nasal endoscopy, which can be performed with a flexible nasofibroscope or a rigid endoscope. Suggestive findings include discoloration or blackening (a sign of ischemia), edema, ulcerations, granulation and crusting of the mucosa, vestibule or columella. In cases of suspected extra-sinusal extension of the disease, paranasal sinus computed tomography is the appropriate tool for proper surgical planning. However, it reveals nonspecific changes in the early phase of the disease, such as unilateral thickening of the nasal cavity or paranasal sinuses. If orbital or cranial invasion is suspected, nuclear magnetic resonance becomes essential.3,11
The definitive diagnosis of AIFRS is established by culture and histopathological evaluation, which can reveal tissue invasion by hyphae.2 Whenever possible, treatment consists of reversing immunosuppression, discontinuing chemotherapy and using granulocyte colony-stimulating factor, combined with antifungal therapy and urgent surgical intervention, which involves aggressive debridement and resection of the entire affected area.3,7
First symptoms of AIFRS are often nonspecific, which contributes to the difficulty in making an early diagnosis, especially among children who are not able to describe their symptoms in detail.2,8 In this study, the two cases occurred in adolescents, both showing increased inflammatory marker C-reactive protein (444 and 135mg/dL) and thrombocytopenia (29,000 and 63,000 platelets/μL). The galactomannan test was only performed on patient P2 (4,12) and, despite decreasing, it remained altered and positive until death.
The main etiological agent described for AIFRS in oncologic and hematological patients is Aspergillus. These microorganisms are saprophytes that are found in decomposed substances, soil and fruits, as well as in the throat, nasal cavities and feces of healthy individuals, but can become pathogenic in immunocompromised patients.2,9 In this report, samples from both cases were sent for culture and the infectious agent Fusarium sp. was identified in both cases. This was also the most commonly isolated fungus in a pediatric series of AIFRS reported by Park et al. and Vinh et al.15,20 However, Ardeshirpour et al. identified Alternaria as the causative agent, whereas Tarkan et al. identified Mucor as the causative agent in eight out of 13 pediatric oncohematological patients.21,22 A similar report by Yakirevitch et al. examined 13 children and identified Mucor (in five patients), Aspergillus (in five patients), Mucor and Aspergillus (in one patient), Exserohilum rostratum (in one patient) and Fusarium (in one patient).23
Fusarium species have emerged as responsible for a broad spectrum of infections, including superficial, locally invasive and disseminated ones, especially in the hospital environment, where reservoirs of infectious species have been reported, especially in the plumbing and water systems.24–27
Fusariosis in immunocompromised patients, mainly children, is usually an invasive and frequently serious disseminated disease, which is the most frequent and challenging clinical form of this infection, accounting for approximately 70% all cases.24 The upper respiratory tract is the main entrance for Fusarium spp., followed by the skin mucosal membranes. Various organs can be affected, including nasal cavities, sinuses, lungs, joints, retina, liver, spleen and kidneys.28,29
The incidence of invasive fusariosis in immunosuppressed patients is variable. An epidemiologic study conducted at eight Brazilian centers between 2007 and 2009 reported a 1-year cumulative incidence of 5.2% among allogeneic hematopoietic cell transplant recipients and of 3.8% in patients with acute myeloid leukemia.30 Incidence of invasive fusariosis here seems higher than that reported in other regions of the world and it is not clear why.
Acute leukemia and T-cell immunodeficiency, in addition to prolonged and deep neutropenia, is one of the main risk factors for invasive fusariosis.31 In our study, the underlying disease was acute myeloid leukemia and severe aplastic anemia and all of the patients presented febrile neutropenia.
Gillespie et al. described the most frequently affected locations as follows (in order of frequency): the middle concha, septum, hard palate, and inferior turbinate.11 Here, discoloration, crusting or granulation were more common findings than ulcerations. Similarly, the most commonly affected locations found by Tarkan et al. were the middle concha and the septum.22 In the present study, the most affected locations were the nasal septum, middle turbinate and inferior turbinate.
A study by Vinh et al. examined all causes of mortality and demonstrated a survival rate of 59% at 30 days and 41% at 6 months.20 Gillespie et al. observed 25 patients, of whom 10 recovered, 9 died due to the disease and 6 died due to other causes.11 On the contrary, Ardeshirpour et al. reported a cure of all 11 patients (100%) without relapse, but three patients eventually died from unrelated causes.21 In our case series, one patient with acute myeloid leukemia was considered cured, with no evidence of recurrent fungal infection after one year of follow-up. The clinical outcome of the patient diagnosed with aplastic anemia was death due to other causes besides RSFIA.
It is recommended that after surgery and while neutropenia is still present, a nasosinusal endoscopic follow-up of AIFRS patients be performed weekly. After neutrophil count recovery, such a follow-up should be performed monthly for an additional six months.31 At our service, this follow-up protocol is performed by the pediatrician and the oncologist who administers the associated systemic antifungal therapy. This study helped define the management protocol that will be followed at this service (Figure 1).
ConclusionIn this chart review, AIFRS was observed in 7.4% of oncohematological pediatric patients assessed with suspected fungal infection at our hospital. They presented with minor local signs of nasal and sinusital inflammation, as well as constitutional signs and symptoms. This demonstrates the importance of fever as a symptom and that it should prompt otorhinolaryngological investigation in immunocompromised patients, especially considering they have a higher potential of fungal infection. Prompt assessment and management are crucial to decrease morbidity and mortality. AIFRS should be considered in all patients with an immunosuppressive disease with fever or nasosinusal symptoms, especially in the presence of septal mucosa and turbinate changes, such as discoloration or blackening, edema, ulcerations, granulation and crusting. We are currently expanding the study population for future studies.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thank Editage (www.editage.com) for English language editing.