Since the outbreak of the new coronavirus pandemic, there have been many questions regarding COVID-19 in patients with SCD, including the pediatric population. A common complication of the disease is ACS, also one of the main causes of death.1
Few data has been published about SARS-CoV-2 infection and ACS in SCD adult patients and until the moment even less cases have been published in the pediatric group. As ACS can be a life-threatening condition, it is important to understand its effects on this specific population.
Patients presentationWe describe below three cases of SCD and ACS that had a positive result for SARS-CoV-2 admitted at a tertiary university affiliated hospital at São Paulo, Brazil.
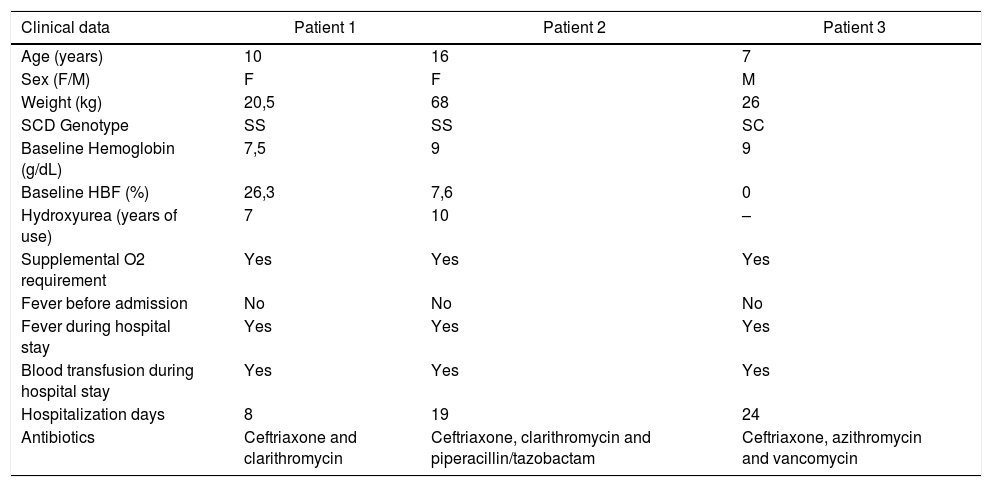
All clinical and laboratory findings of the three patients are described in Tables 1 and 2.
Clinical data.
| Clinical data | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Age (years) | 10 | 16 | 7 |
| Sex (F/M) | F | F | M |
| Weight (kg) | 20,5 | 68 | 26 |
| SCD Genotype | SS | SS | SC |
| Baseline Hemoglobin (g/dL) | 7,5 | 9 | 9 |
| Baseline HBF (%) | 26,3 | 7,6 | 0 |
| Hydroxyurea (years of use) | 7 | 10 | – |
| Supplemental O2 requirement | Yes | Yes | Yes |
| Fever before admission | No | No | No |
| Fever during hospital stay | Yes | Yes | Yes |
| Blood transfusion during hospital stay | Yes | Yes | Yes |
| Hospitalization days | 8 | 19 | 24 |
| Antibiotics | Ceftriaxone and clarithromycin | Ceftriaxone, clarithromycin and piperacillin/tazobactam | Ceftriaxone, azithromycin and vancomycin |
Laboratorial markers.
| Laboratorial markers | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Initial Hemoglobin (g/dL) | 6,9 | 9,1 | 10,5 |
| First CRP (mg/L) | None | 1,23 | 296 |
| Maximum CRP (mg/L) | None | 277,43 | 296 |
| First D- dimer (mcg/mL) | 3,57 | 5,83 | 5,99 |
| Maximum D- dimer (mcg/mL) | 4,88 | 12,93 | 7,73 |
| Initial leucocyte (mcg/L) | 12135 | 13518 | 30320 |
| Troponin (pg/mL) | <5 | <5 | <5 |
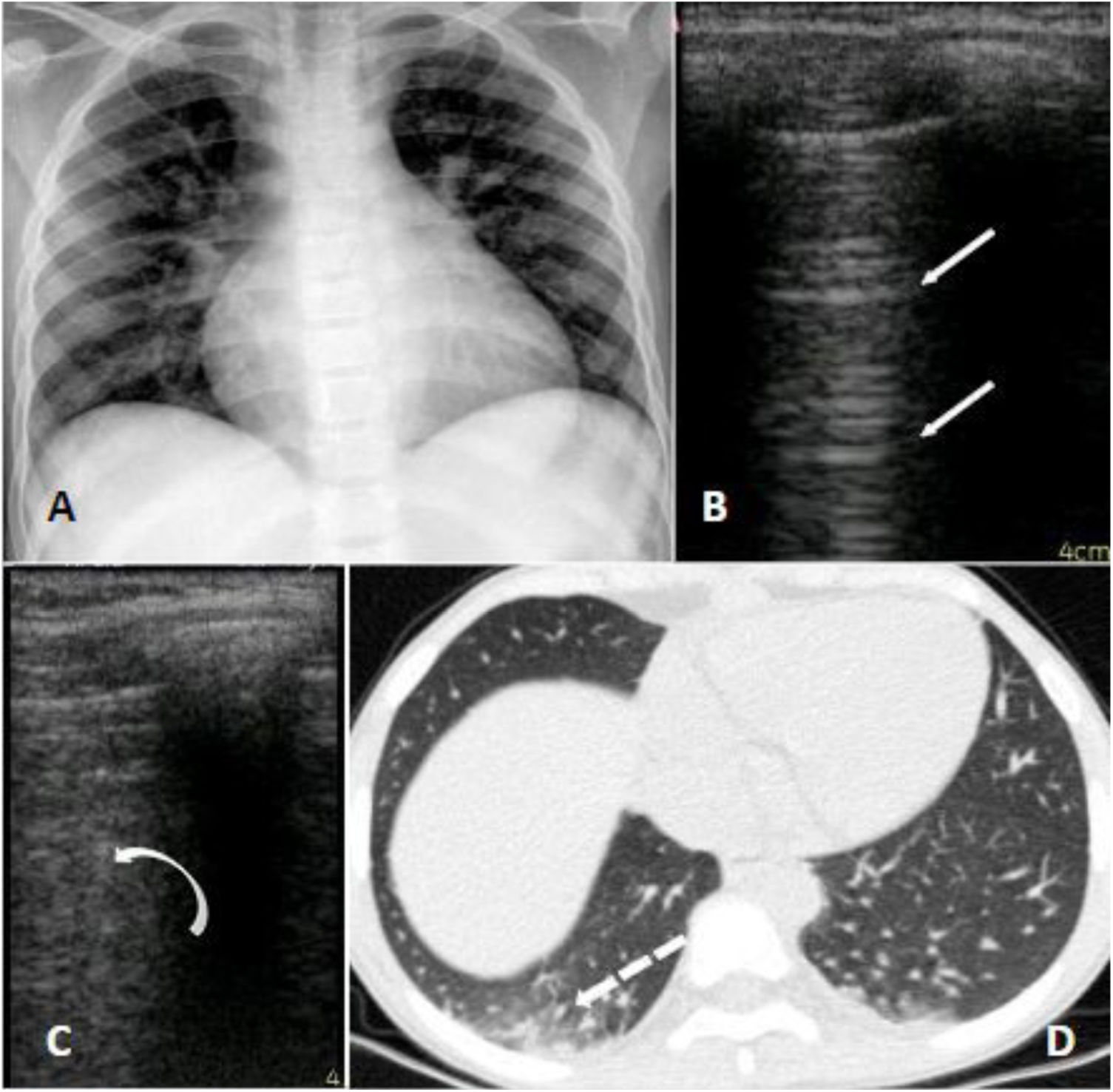
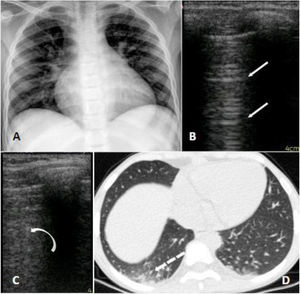
A 10-year-old girl with homozygous SCD checked in at the hospital with neck pain starting 8 h before admission. She had a past history of multiple hospitalizations due to painful VOC and ACS, for which she started the use of hydroxyurea at the age of four. She had no respiratory symptoms or fever whatsoever and was initially treated with morphine and intravenous fluids. At day 2 she presented a SpO2 of 90% and temperature of 38 °C, supplemental oxygen was indicated, infectious screening was performed with blood exams, cultures, chest radiograph and nasopharyngeal swab for SARS-CoV-2, and ceftriaxone and clarithromycin were initiated. On day 3 she received red blood transfusion and COVID-19 was confirmed. The radiographic findings were unspecific: a mild cardiomegaly due to chronic anemia and subtle bilateral peribronchial infiltrate; a chest CT was performed which showed basal bilateral infiltrates with small ground-glass opacities associated with areas of consolidation. The LUS identified the presence of multiples B lines on the inferior lobes of both hemithoraces (Fig. 1). She was afebrile after day 4 and no supplemental oxygen was required after day 5. Her laboratory exams were stable, all cultures were negative, and she was discharged on day 8.
Panel A (radiograph): mild infiltrate peribronchial; Panel B (LUS): normal pulmonary aeration; A lines on left superior lobe. Panel C (LUS): B lines which correspond to alveolar-interstitial syndrome of right inferior lobe. Panel D (CT): peripheral small ground glass consolidations on the right inferior lobe.
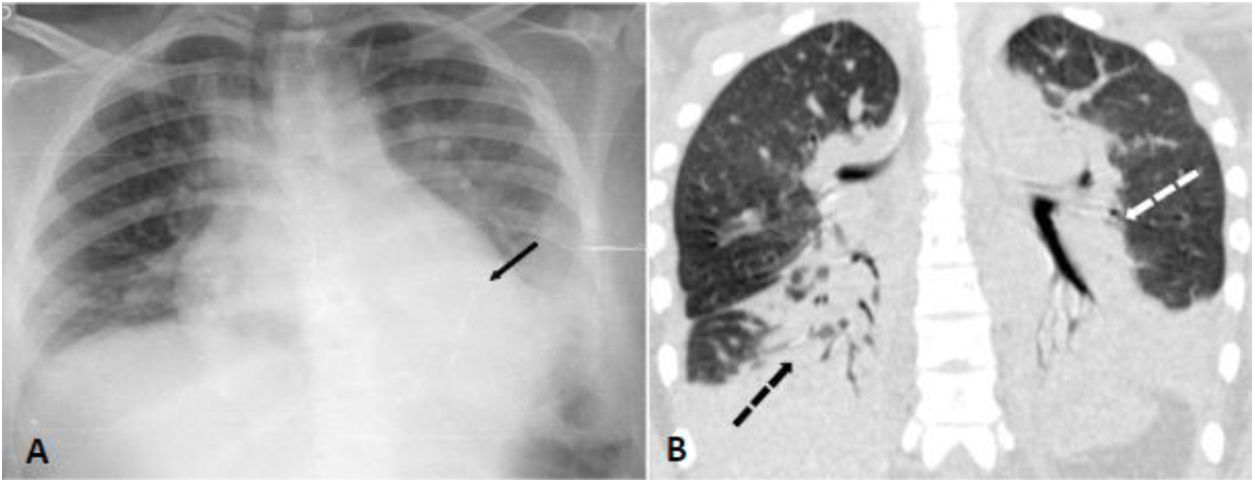
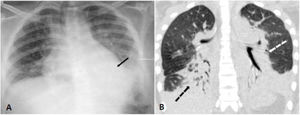
A 16-year-old female homozygous SCD, was admitted at the hospital with 12 h of abdominal, back and leg pain. She was in use of hydroxyurea since the age of six due to multiple episodes of ACS and VOC. The patient received morphine and intravenous fluids and at day 2 presented SpO2 of 88% so nasopharyngeal swab for SARS-CoV-2 was performed. Bilateral basal consolidations were found in the chest radiograph (Fig. 2), ceftriaxone, clarithromycin and supplementary oxygen were initiated. COVID-19 infection was confirmed and chest CT showed consolidations, infiltrates with ground-glass opacities and small pleural effusion on both lungs (Fig. 2). After 72 h of antibiotics she became afebrile but on day 7 and 8 she presented an axillary temperature of 38,5 °C and antibiotics were changed to piperacillin-tazobactam. On day 10 nasopharyngeal swab for SARS-CoV-2 was negative, she was afebrile after day 12 and all blood cultures were negative. She was dismissed after 19 days of hospitalization.
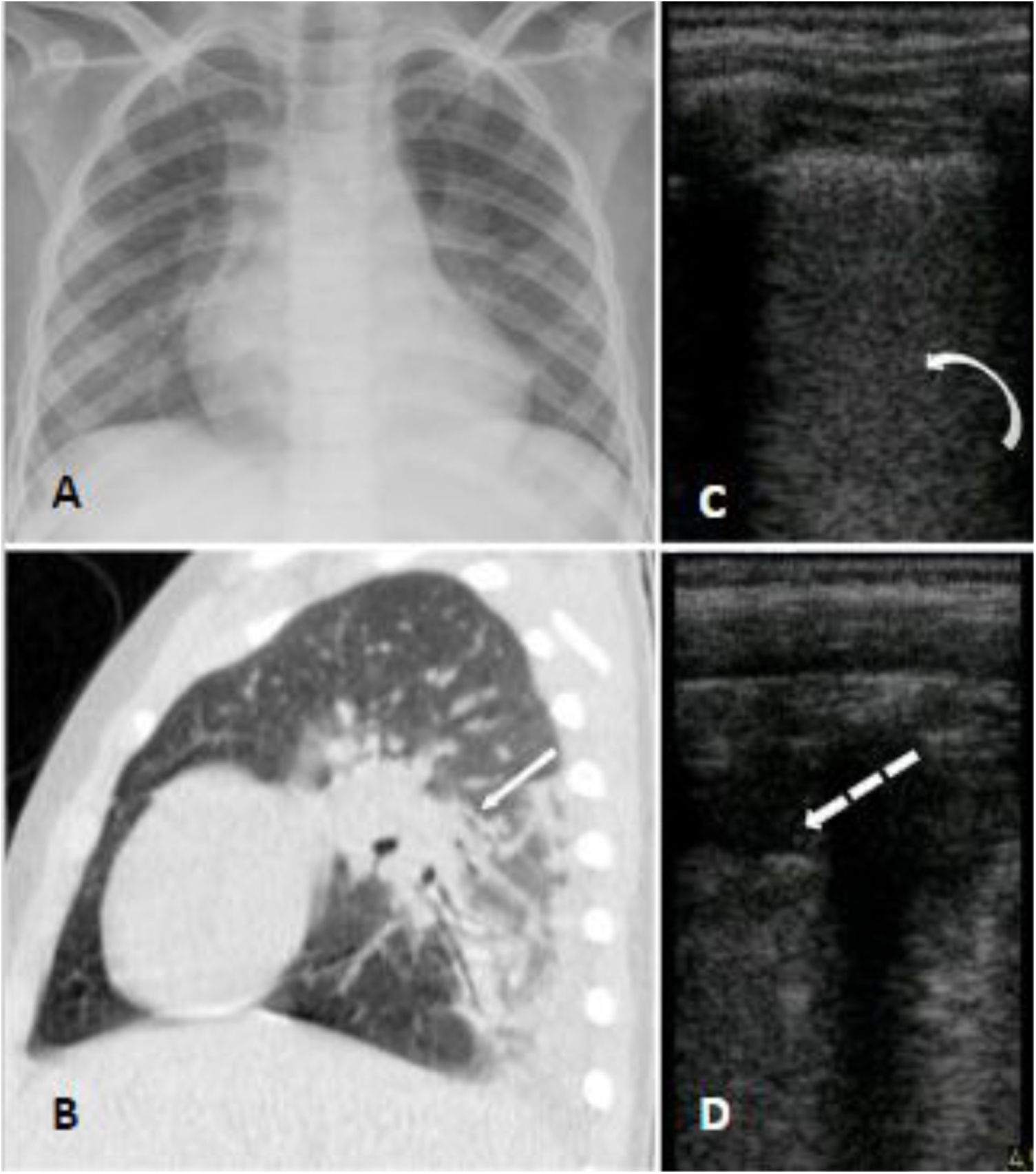
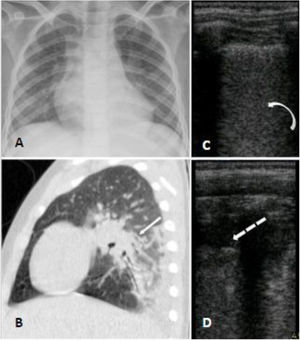
Case 3A seven-year-old boy with hemoglobin SC disease was admitted at the hospital with four days of abdominal and leg pain and dry cough. He reported no fever but was in use of regular ibuprofen and dipyrone for pain control for the last days. He had a past history of asthma which required continuous use of beclomethasone inhalation. At hospital his SpO2 was 90% on room air and he was admitted for supplemental oxygen, ceftriaxone and azithromycin, as well as morphine and intravenous fluids. Nasopharyngeal swab for SARS-CoV-2 was performed and the virus was confirmed by RT-PCR. Chest radiograph showed bilateral patchy consolidations and chest CT showed areas of air space consolidation and ground-glass opacity in association with atelectasis on both lungs; the ultrasonographic findings were coalescent B lines, subpleural consolidations and thickened pleural line on inferior zones of both lungs (Fig. 3). At this occasion d- dimer was 5,99 mcg/mL. At day 6 he became febrile, and on day 8 began salbutamol and dexamethasone for asthma exacerbation. He maintained the need for supplemental oxygen and regular antipyretic, new chest CT showed worse pulmonary images - the consolidations were larger and the distribution of the ground glass opacities were diffuse - and laboratory exams showed platelets of 1.289.000/uL and d-dimer of 7,7 mcg/mL, so enoxaparin and vancomycin were initiated. He later showed epigastric pain, an echocardiogram and a CT pulmonary angiography for thromboembolism protocol were performed, both with negative results. After day 13 he was afebrile and required no more supplemental oxygen. Cultures showed no bacterial growth and nasopharyngeal swab for SARS-CoV-2 was negative on day 23. He completed a total of 24 days of hospitalization.
Panel A (chest radiograph): retrocardiac bilateral peribronchial consolidations. Panel B (sagittal chest CT): peri bronchial patchy consolidations and ground glass consolidations on the left lung. Panel C and D (LUS): multiple B lines erasing A lines and subpleural peripheral consolidation implying alveolar-interstitial syndrome of inferior zones of both lungs.
From March 2020 until July 2020, 38 pediatric patients with SCD were admitted at the university´s Hospital and were tested for SARS-CoV-2 by RT-PCR, of whom six had a positive result. None of those patients required treatment in ICU and only three presented with ACS.
The three patients were admitted in our service with VOC and only the patient of case number three had flu-like symptoms (dry cough). During hospitalization, all presented fever and desaturation, typical symptoms of both COVID-19 infection and ACS. We are still learning about the manifestations of SARS-CoV-2 in SCD patients, however the initial presentation of COVID-19 as painful VOC and then evolving with ACS has already been seen in adults.2,3 Viral infections are well established as triggers to these SCD acute manifestations4,5 and the one caused by the new coronavirus might not be an exception.
All patients needed symple blood transfusion, antibiotic therapy and supplemental oxygen via nasal cannula. Patients 1 and 2 had sickle cell anemia and no other comorbidities, presented good clinical course and needed no additional therapies. In our service SCD pediatric patients do not receive prophylactic anticoagulation as protocol, however in patient number 3 this therapy was necessary due to clinical and laboratory worsening.
An important difference between the children of the group is that patient number three had SC genotype and asthma, factors that might corroborate the worse clinical course. There is still not enough data to confirm differences in complications or mortality in patients with different SCD genotypes infected with the new coronavirus nor the implications of asthma,
although pulmonary disease have already been described as related factor for worse prognosis in COVID-19,6 and as ACS trigger in SCD children.7
In our series of cases, only patient of case number three did not use hydroxyurea. Previous literature has already established its use in the pediatric population due to its beneficial effects on lowering chronic complications as well as the number of acute episodes such as VOC and ACS,8–10 but there is still no consensus whether it might predict a positive outcome in SCD patients in this new scenario.11 Despite that, it is important to note that the patient who did not use the medication had a more complicated clinical course.
Although COVID-19 has been reported to be less severe among children, the radiologic findings on the three cases above are similar to the ones described in adults.12 The overlap of ACS and lung disease caused by SARS-CoV-2 is possibly the explanation for more significant radiological findings in this group.
When compared with literature,13 our patients did not differ in terms of genotype, time of hospitalization and clinical outcome. No patients in this study died, needed ICU nor mechanical ventilation. It is still unknown the reason why these patients are showing a better clinical evolution than previously predicted.
ConclusionPulmonary viral infections, such as the one caused by SARS-CoV-2, can predispose SCD patients to painful VOC and ACS, and generate greater need for hospitalization. It is important to note that the COVID-19 symptoms, such as fever, desaturation and dyspnea, are often similar to the symptoms of ACS and may affect clinical decisions. Since data on patients with SCD infected with the new coronavirus is scarce, especially in the pediatric group, it is necessary to obtain more information in order to reduce the uncertainties still present in medical practice.