Thrombocytopenia (TP) is the major event associated with linezolid (LZD) therapy. We investigated the incidence and risk factors for thrombocytopenia in hospitalized adults who received LZD (1200mg/day) between 2015 and 2017. HIV-positive, death during follow-up and those with a baseline platelet count ≤100×103/mm3 were excluded.
MethodTP was defined as a decrease in platelet count of ≥20% from the baseline level at the initiation of linezolid therapy and a final count of <100×103/mm3. The odds ratios (OR) for thrombocytopenia were obtained using multivariate stepwise logistic regression analysis.
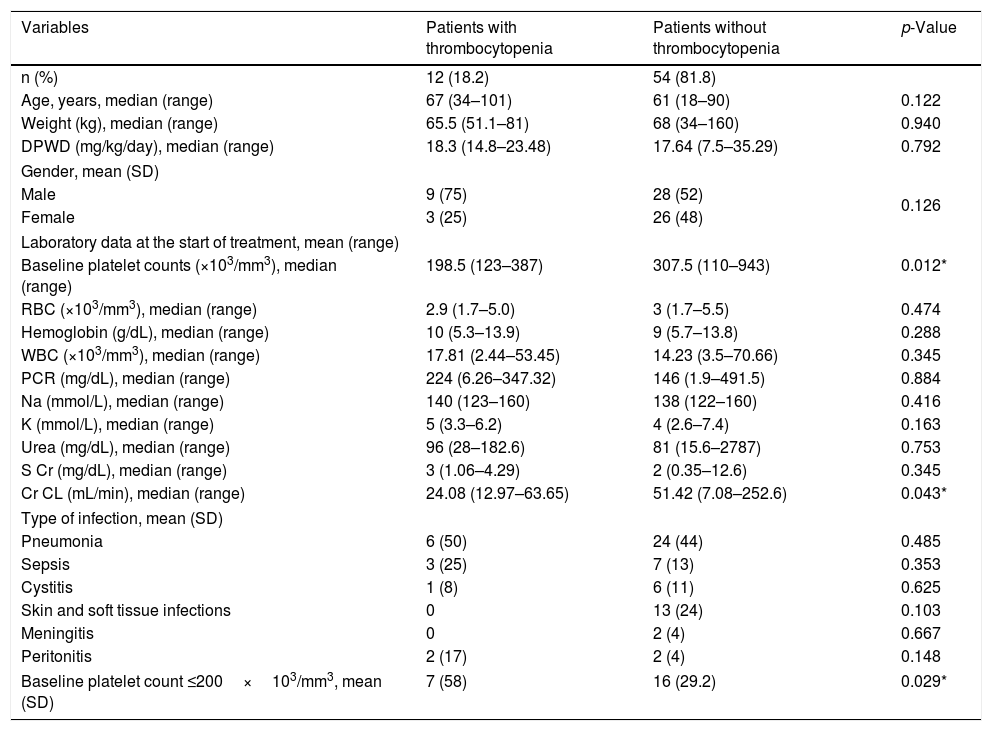
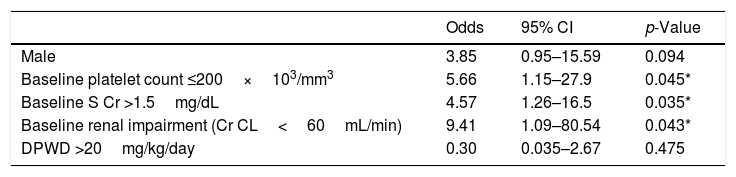
Main resultsA total of 66 patients were included (mean age [SD] 62 [18], male gender [%], 37 [56]). LZD-associated TP was identified in 12 patients (18.2%). For TP, the adjusted OR [95% CI] of the platelet count ≤200×103/mm3, serum creatinine and renal impairment at baseline were 5.66 [1.15–27.9], 4.57 [1.26–16.5] and 9.41 [1.09–80.54], respectively. Male gender and dosage per weight per day (DPWD) >20mg/kg/day were not risk factors.
ConclusionThe results showed that the incidence of linezolid-induced thrombocytopenia was lower in patients with normal renal function and higher in those with platelet counts ≤200×103/mm3 or serum creatinine >1.5mg/dL at the start of the treatment.
Linezolid (LZD) is an antibiotic of the oxazolidinone class that is used for the treatment of infections caused by Gram-positive bacteria, including those due to methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VRE). It inhibits protein synthesis by binding to a site on the bacterial 23s ribosomal RNA of the 50s subunit, which is essential to the replication process.1 LZD use should be restricted as an alternative treatment for infections caused by multi-resistant microorganisms and should not be used when there are other effective drugs. The inadequate use of LZD could increase the selection of resistant strains, resulting in the loss of effectiveness of this antibiotic.2
The recommended dose in adults is 600mg, administered orally or intravenously twice daily. The elimination half-life is approximately 5.5h, the maximum serum concentration (Cmax) ranges from 15 to 21μg/mL, and the time to reach the Cmax via oral intake, is approximately 1.5h. It is not recommended to adjust the dose for patients with renal dysfunction.3,4 However, the antimicrobial concentration is higher in patients with renal impairment, and current studies have indicated that renal impairment is a risk factor for the development of linezolid-induced thrombocytopenia.5–7
Although linezolid use has advantages, it has been associated with adverse effects such as gastrointestinal disorders, hematological toxicity and cutaneous reactions.8,9 The hematological reactions include anemia, eosinophilia, myelosuppression, neutropenia, cell aplasia and thrombocytopenia.10 The normal peripheral platelet count is between 150 and 450×103/mm3. Thrombocytopenia has been defined as a platelet count <150×103/mm3, with or without spontaneous bleeding, ecchymosis or petechiae.11 Thrombocytopenia can result from decreased platelet production, increased platelet consumption, or sequestration. In this sense, linezolid-induced thrombocytopenia may be the most common cause of platelet destruction, whether by immune-mediated reaction or by myelosuppression.12 Some clinical studies have reported events linking linezolid therapy to thrombocytopenia development. For example, Tajima et al. suggested that LDZ use for more than 14 days, renal dysfunction and chronic liver disease were risk factors linked to linezolid-induced thrombocytopenia.9 In addition, a low baseline platelet count and body weight were associated with a decrease in the peripheral platelet count.13,14 However, the main mechanism that regulates the pathogenesis of this adverse effect is still obscure.
In response to the reports on this topic and the occurrence of such adverse effects associated with the use of linezolid, which may cause life-threatening risks to the patients, we performed this retrospective cohort study. Despite several case reports in Brazil, this is the first cohort study using this approach. Here, we aimed to identify the incidence and the risk factors, with focus on renal function, for linezolid-induced thrombocytopenia in adult patients hospitalized in the Midwestern Region of Brazil.
MethodsStudy designThis retrospective cohort study was conducted between March 2015 and March 2017 at the Hospital Universitário Maria Aparecida Pedrossian of the Universidade Federal de Mato Grosso do Sul (HUMAP-UFMS, 242 beds). This study was reviewed and approved by the ethics committee of the HUMAP hospital (CAAE 79967117.0.0000.0021).
Studied populationWe examined hospitalized patients >18 years old treated with a daily dose of 1200mg by IV (600mg every 12h) of linezolid for 10 days. HIV-positive patients, patients who died during treatment or the follow-up period and those that presented with a baseline platelet count ≤100×103/mm3 were excluded from the study. Laboratory and clinical data were extracted from electronic medical records and stored in a Microsoft Excel file, without identifying patient names and/or numbers.
Thrombocytopenia, data analysis and risk factorsThrombocytopenia was defined as a decrease in the platelet count of ≥20% from the baseline level at the initiation of linezolid therapy and a final count of <100×103/mm3, based on the previous report.6 Baseline platelets count was defined as the platelet count prior to the initiation of linezolid therapy. In addition, by using the criteria proposed by George et al.,15 we were able to establish the clinical evidence for a causal relation between LZD and thrombocytopenia. The definition of levels (I, II, III and IV) of evidence were described previously by the authors.15
The thrombocytopenia severity grade was evaluated using the platelet count range at baseline, according to the Common Terminology Criteria for Adverse Events v4.0 (CTCAE v4.0), developed by the National Cancer Institute (NCI) and adapted by Hirano et al.6: grade 1 (100−75×103/mm3), grade 2 (74−50×103/mm3), grade 3 (49−25×103/mm3), grade 4 (<25×103/mm3).
All patients received a standard total daily dose of 1200mg of linezolid, however, no adjustments of dose per body weight were considered.
The creatinine clearance (Cr CL) was estimated using the Cockcroft-Gault equation [Cr CL (mL/min)=(140−age)×(weight/72)/serum creatinine (mg/mL)], and for females this was multiplied by 0.85.16 To compare the relationship between renal function and the incidence of linezolid-induced thrombocytopenia, we grouped the patients according to their creatinine clearance. Patients with Cr CL rates of less than 30, 30–60, or greater than 60mL/min, were classified as insufficient renal function,6 mildly insufficient renal function or normal renal function, respectively.
The five variables: male, baseline platelet count ≤200×103/mm3, baseline serum creatinine >1.5mg/dL, baseline renal impairment (Cr CL<60mL/min) and dosage per weight per day (DPWD) >20mg/kg/day were analyzed by logistic regression analysis as risk factors associated with thrombocytopenia in patients who received linezolid during the follow-up.
Statistical analysisFisher’s exact test was used for comparisons; the Mann–Whitney test was the nonparametric alternative, and; the Student’s t-test was used for variables with non-normal distributions. The multivariate stepwise logistic regression analysis was performed to determine the odds ratios (OR) for thrombocytopenia. Resulting p-values <0.05 were considered significant. All analyses were performed according to BioEstat version 5.0 tutorials (https://www.mamiraua.org.br/pt-br/downloads/programas/bioestat-versao-53/).
Ethics approval and consent to participateThe study was in accordance with the ethical standards of the declaration of Helsinki and approved by the local ethics committee of the HUMAP hospital (CAAE 79967117.0.0000.0021). Given the retrospective data analysis nature, informed consent was not required.
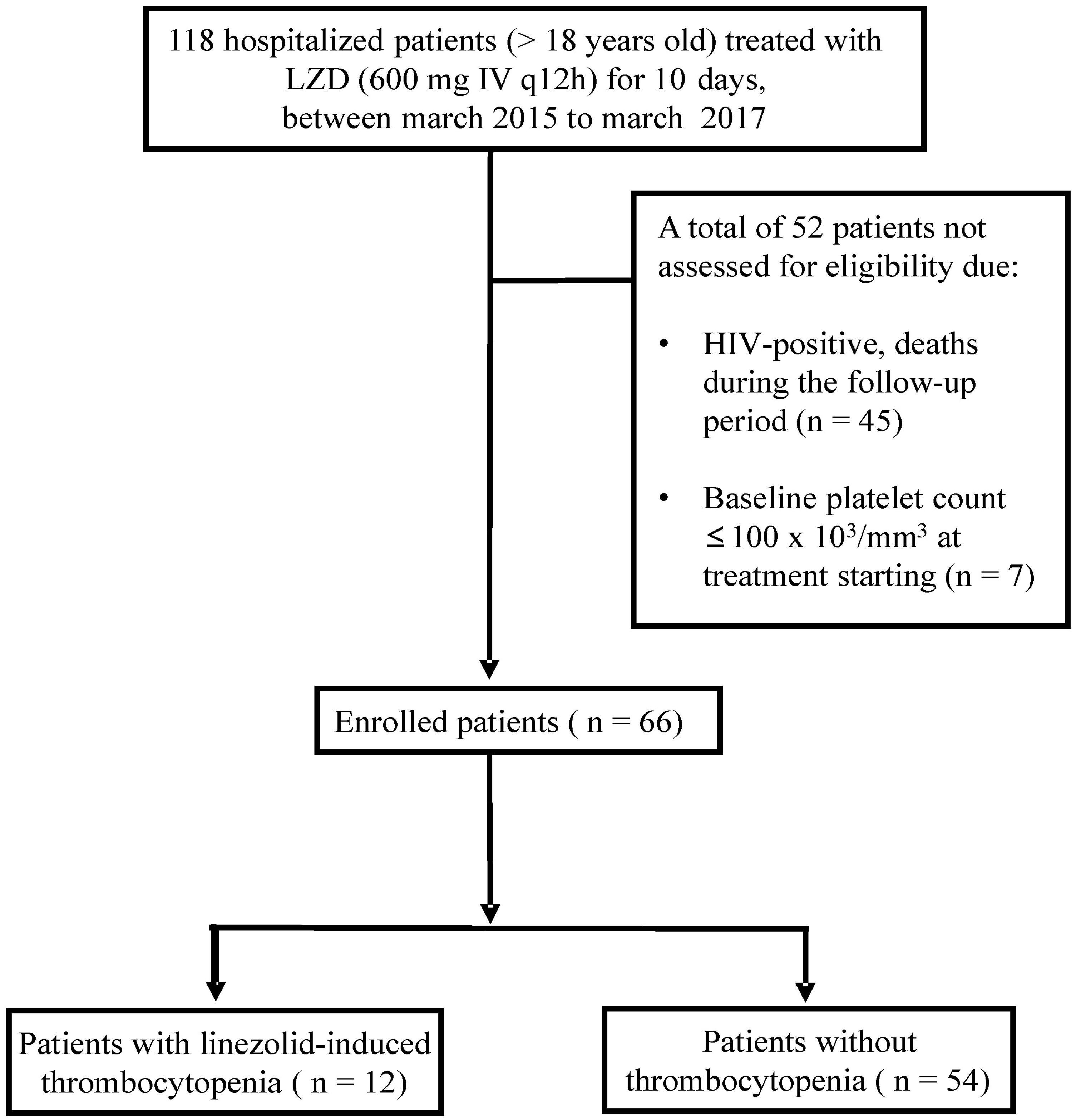
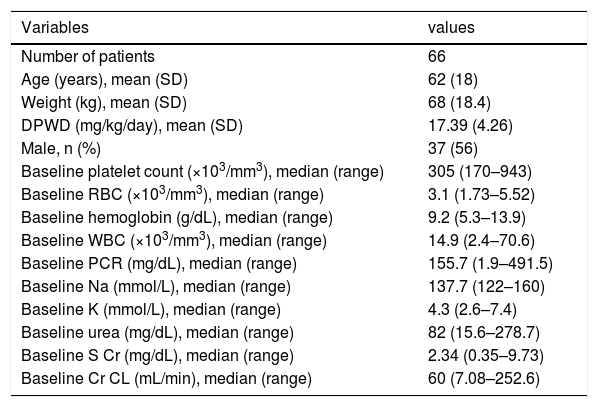
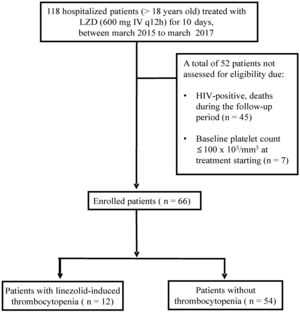
ResultsA total of 66 patients, being 37 males and 29 females of mean age (SD) 62 (18) years were included in this study (Figure 1). The patients´ mean (SD) body weight at admission was 68 (18.4) kg and the DPWD of linezolid was 17.39 (4.26) mg/kg/day. The patient characteristics at baseline are listed in Table 1.
Patient characteristics at baseline (2015–2017).
| Variables | values |
|---|---|
| Number of patients | 66 |
| Age (years), mean (SD) | 62 (18) |
| Weight (kg), mean (SD) | 68 (18.4) |
| DPWD (mg/kg/day), mean (SD) | 17.39 (4.26) |
| Male, n (%) | 37 (56) |
| Baseline platelet count (×103/mm3), median (range) | 305 (170–943) |
| Baseline RBC (×103/mm3), median (range) | 3.1 (1.73–5.52) |
| Baseline hemoglobin (g/dL), median (range) | 9.2 (5.3–13.9) |
| Baseline WBC (×103/mm3), median (range) | 14.9 (2.4–70.6) |
| Baseline PCR (mg/dL), median (range) | 155.7 (1.9–491.5) |
| Baseline Na (mmol/L), median (range) | 137.7 (122–160) |
| Baseline K (mmol/L), median (range) | 4.3 (2.6–7.4) |
| Baseline urea (mg/dL), median (range) | 82 (15.6–278.7) |
| Baseline S Cr (mg/dL), median (range) | 2.34 (0.35–9.73) |
| Baseline Cr CL (mL/min), median (range) | 60 (7.08–252.6) |
| Type of infection, n (%) | |
|---|---|
| Pneumonia | 30 (45) |
| Sepsis | 10 (15) |
| Cystitis | 7 (11) |
| Skin and soft tissue infections | 13 (20) |
| Meningitis | 2 (3) |
| Peritonitis | 4 (6) |
The data are presented as n (%), median (range) or mean (SD); SD standard derivation, DPWD dosage per weight per day, RBC red blood cells, WBC white blood cells, PCR protein C reactive, S Cr serum creatinine, Cr CL clearance creatinine.
The linezolid was mainly administered for pneumonia (30/66) and sepsis (10/66), and among the treatment courses, 36 (54.5%) were administered to patients in the intensive care unit. At the start of the linezolid treatment, 36 patients (54.5%) had impaired renal function (Cr CL<60mL/min). The median (range) of baseline S Cr and Cr CL was 2.34mg/dL (0.35–9.73) and 60mL/min (7.08–252.6), respectively.
Thrombocytopenia occurred in twelve adult patients treated with linezolid (18.2%), of whom five (42%) required platelet transfusions during follow-up. Our results supported probable (58%) and possible evidence (42%) for a causal relation of LZD with the occurrence of thrombocytopenia, using the proposed criteria.
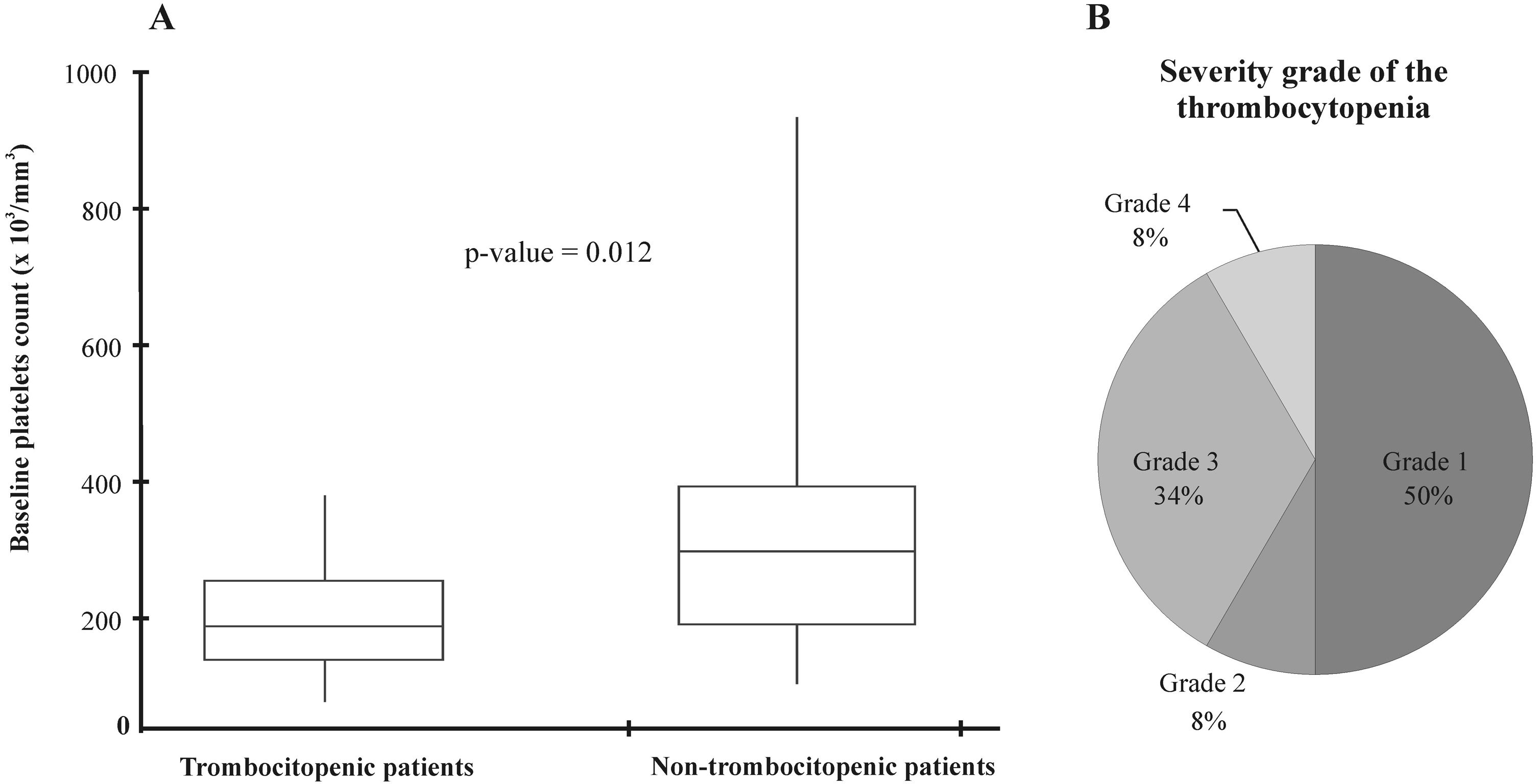
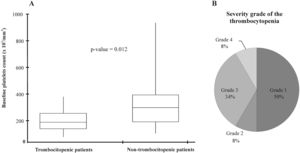
The median platelet count at treatment initiation was significantly lower in patients who developed thrombocytopenia (Figure 2A). Regarding the thrombocytopenia severity grade, five patients (42%) were categorized as ≥grade 3, according to the criteria proposed in methods (Figure 2B). Subsequently, the characteristics of thrombocytopenic and non-thrombocytopenic patients were compared. As shown in Table 2, the patients with thrombocytopenia presented with lower platelet counts at baseline than those without thrombocytopenia (p= 0.012). Additionally, linezolid-induced thrombocytopenia in patients with an initial platelet count ≤200×103/mm3 was seen in 58% (7/12) of the patients (p=0.029).
Background data of the patient characteristics with and without thrombocytopenia (2015–2017).
| Variables | Patients with thrombocytopenia | Patients without thrombocytopenia | p-Value |
|---|---|---|---|
| n (%) | 12 (18.2) | 54 (81.8) | |
| Age, years, median (range) | 67 (34–101) | 61 (18–90) | 0.122 |
| Weight (kg), median (range) | 65.5 (51.1–81) | 68 (34–160) | 0.940 |
| DPWD (mg/kg/day), median (range) | 18.3 (14.8–23.48) | 17.64 (7.5–35.29) | 0.792 |
| Gender, mean (SD) | |||
| Male | 9 (75) | 28 (52) | 0.126 |
| Female | 3 (25) | 26 (48) | |
| Laboratory data at the start of treatment, mean (range) | |||
| Baseline platelet counts (×103/mm3), median (range) | 198.5 (123–387) | 307.5 (110–943) | 0.012* |
| RBC (×103/mm3), median (range) | 2.9 (1.7–5.0) | 3 (1.7–5.5) | 0.474 |
| Hemoglobin (g/dL), median (range) | 10 (5.3–13.9) | 9 (5.7–13.8) | 0.288 |
| WBC (×103/mm3), median (range) | 17.81 (2.44–53.45) | 14.23 (3.5–70.66) | 0.345 |
| PCR (mg/dL), median (range) | 224 (6.26–347.32) | 146 (1.9–491.5) | 0.884 |
| Na (mmol/L), median (range) | 140 (123–160) | 138 (122–160) | 0.416 |
| K (mmol/L), median (range) | 5 (3.3–6.2) | 4 (2.6–7.4) | 0.163 |
| Urea (mg/dL), median (range) | 96 (28–182.6) | 81 (15.6–2787) | 0.753 |
| S Cr (mg/dL), median (range) | 3 (1.06–4.29) | 2 (0.35–12.6) | 0.345 |
| Cr CL (mL/min), median (range) | 24.08 (12.97–63.65) | 51.42 (7.08–252.6) | 0.043* |
| Type of infection, mean (SD) | |||
| Pneumonia | 6 (50) | 24 (44) | 0.485 |
| Sepsis | 3 (25) | 7 (13) | 0.353 |
| Cystitis | 1 (8) | 6 (11) | 0.625 |
| Skin and soft tissue infections | 0 | 13 (24) | 0.103 |
| Meningitis | 0 | 2 (4) | 0.667 |
| Peritonitis | 2 (17) | 2 (4) | 0.148 |
| Baseline platelet count ≤200×103/mm3, mean (SD) | 7 (58) | 16 (29.2) | 0.029* |
The data are presented as n (%), median (range or confidence interval) and mean (SD). The statistical analyses were performed by Mann-Whitney or Student’s t-test using the obtained coefficient of variation (CV) from the descriptive analysis. The Fisher´s exact test was used for comparisons of type of infection. SD standard derivation, DPWD dosage per weight per day, RBC red blood cells, PCR protein C reactive, S Cr serum creatinine, Cr CL clearance creatinine. Differences were considered significant for p-values <0.05 (*).
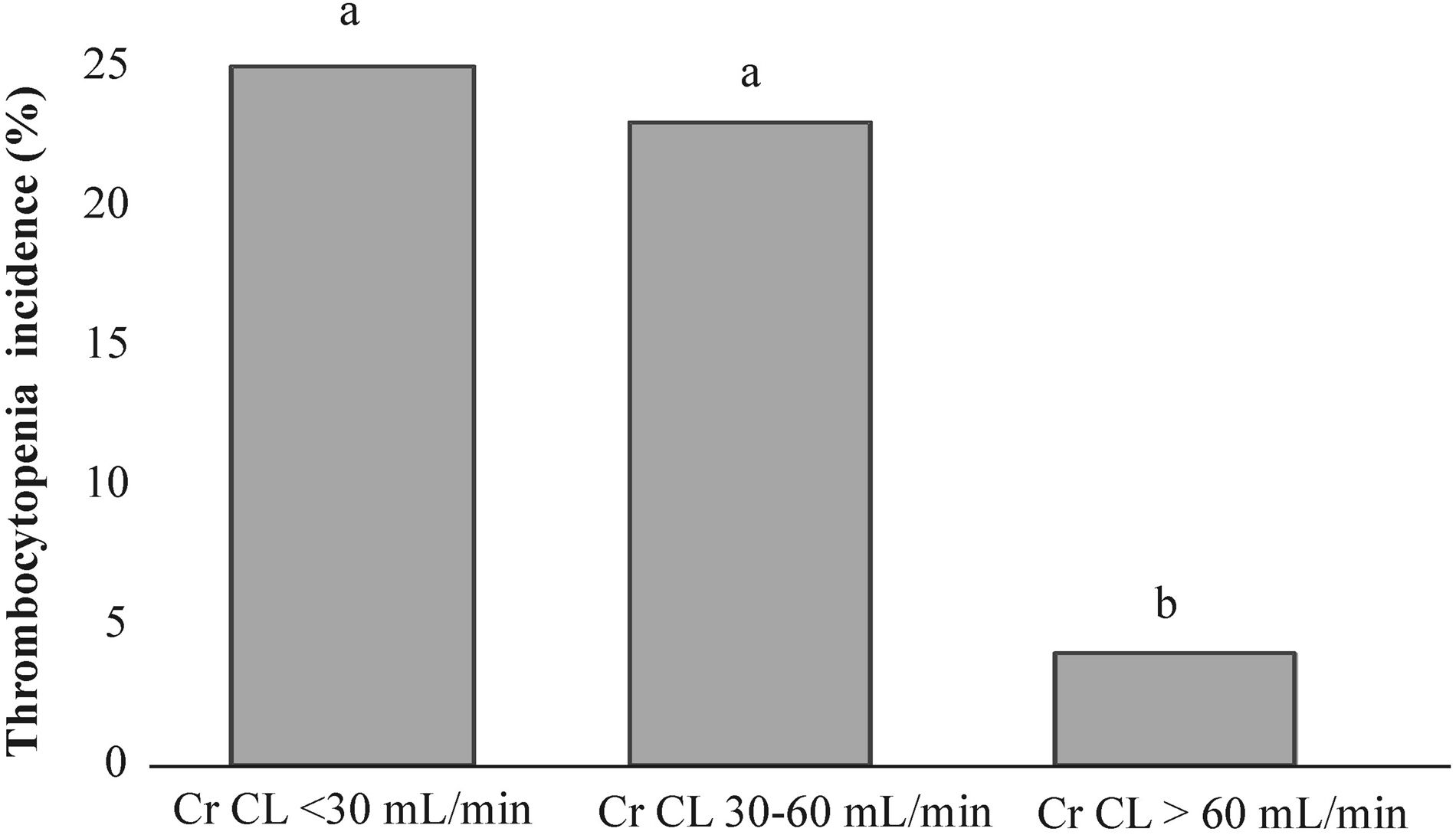
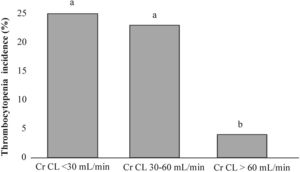
The median of baseline creatinine clearance (Cr CL) was significantly lower in thrombocytopenic patients (p=0.043) (Table 2). When patients were categorized intro three groups according to the sufficiency of their renal function at the start of linezolid treatment (Table 3), the renal insufficient group was significantly associated with thrombocytopenia (p=0.046), while the non-thrombocytopenic patients presented with normal renal function (p=0.016). In addition, Figure 3 presents the comparison of thrombocytopenia incidences in each renal function group, which were significantly higher in insufficient (25%, p=0.041) and mildly insufficient (23%, p=0.034) renal function than among patients with normal renal function (4%).
Renal function evaluation in thrombocytopenic and non-thrombocytopenic patients.
| Patients with thrombocytopenia | Patients without thrombocytopenia | p-Value | |
|---|---|---|---|
| Cr Cl <30mL/min | 6 (60) | 16 (32) | 0.047* |
| Cr Cl 30–60mL/min | 3 (30) | 11 (22) | 0.292 |
| Cr Cl >60mL/min | 1 (10) | 23 (46) | 0.017* |
The data are presented as number (%) and statistical analysis performed by two binomial proportions test. The significant difference p-value <0.05 (*). Cr CL clearance creatinine.
The thrombocytopenia incidence (%) in each renal function group. The patients were stratified according to their creatinine clearance rates into three groups: insufficient renal function (Cr CL<30mL/min), mildly insufficient renal function (Cr CL 30–60mL/min) and normal renal function (Cr CL>60mL/min). The lower-case letters (a) between bars indicate no significant differences, while statistically significant differences are presented by different lower-case letters (a and b) above the bars (binomial test, p<0.05).
Finally, as shown in Table 4, a platelet count ≤200×103/mm3 (OR=5.66; 95% CI, 1.15–27.9; p=0.045), serum creatinine >1.5mg/dL (OR=4.57; 95% CI, 1.26–16.5; p=0.035) and renal impairment (OR=9.41; 95% CI, 1.09–80.54; p=0.043) at the treatment start were considered significant risk factors for linezolid-associated thrombocytopenia by statistical analysis.
Risk factors for linezolid-induced thrombocytopenia in hospitalized adult patients.
| Odds | 95% CI | p-Value | |
|---|---|---|---|
| Male | 3.85 | 0.95–15.59 | 0.094 |
| Baseline platelet count ≤200×103/mm3 | 5.66 | 1.15–27.9 | 0.045* |
| Baseline S Cr >1.5mg/dL | 4.57 | 1.26–16.5 | 0.035* |
| Baseline renal impairment (Cr CL<60mL/min) | 9.41 | 1.09–80.54 | 0.043* |
| DPWD >20mg/kg/day | 0.30 | 0.035–2.67 | 0.475 |
OR odds ratio, CI confidence interval, S Cr serum creatinine, Cr CL creatinine clearance calculated by Cockcroft–Gault equation, DPWD dosage per weight per day. Differences were considered significant for p-values <0.05(*).
In general, the drug linezolid is well tolerated, but its use could be limited by the potential risk of thrombocytopenia, attributable to reversible bone marrow suppression.12 Linezolid-induced thrombocytopenia has been previously reported in 2.4–66% of patients who receive LZD therapy.6,8,17–22 In the present study, we found an incidence of 18.2% in adult patients hospitalized in the Brazilian Midwest.
Several studies describe risk factors associated with the development of thrombocytopenia in patients who received linezolid therapy. For example, Takahashi et al. reported that the oral administration of linezolid decreased the risk of thrombocytopenia, while the duration of treatment (≥14 days), creatinine clearance (<50mL/min) and chronic liver disorders were associated with higher risk.18 In addition, Hirano et al. described individuals with creatinine clearance rates <30mL/min having a significantly higher incidence of thrombocytopenia than those with Cr CL greater than 60mL/min.6 Other risk factors also have been reported, such as age, gender, body weight, daily dose per kg, serum creatinine, hemodialysis, platelet count at baseline, and anemia.5,7,19,23
Our data showed that the incidence of linezolid-induced thrombocytopenia was lower in patients with normal renal function. Although the renal function had little effect on the overall clearance of linezolid, the accumulation of metabolites produced by linezolid oxidation (2-aminoethoxy-acetate and hydroxyethyl-glycine analogue) has been reported in patients with renal dysfunction as possibly associated with this toxicity, but this remains unclear.24 Tsuji et al. speculated that both metabolites induce injury to the liver, causing an increase in the linezolid serum concentration due the inhibition of its transfer to hepatocytes.25 Rabon et al. associated severe renal dysfunction and hepatic dysfunction to thrombocytopenia development during LZD treatment.23 Taken together, these events correlate with the adverse hematological events, such as peripheral platelet count reduction, with reduced peripheral platelet counts. In the literature, several reports have investigated the relationship between Cr CL rates and linezolid clearance in patients with kidney failure, reinforcing the association with linezolid accumulation and toxicity.26,27
Rayner et al., through pharmacokinetic and pharmacodynamic relationships, suggested that twice-daily 600mg linezolid dosing might be considered an overdose in the treatment of elderly or adult patients with renal impairment.28 The authors explained that this could be due to a decrease in linezolid clearance rates, as shown by the AUC (area under curve) analysis, which represents the duration and the degree of exposure to linezolid; an increase in the AUC was found in these patients, culminating in the development of hematological alterations.
Matsumoto et al. (2010) quantified the plasma concentrations of linezolid by chromatography and demonstrated a correlation between impaired linezolid clearance and lower renal filtration rates. In this sense, platelet count monitoring had been recommended in patients treated with linezolid for longer than 7 days, especially for those with renal impairment, because of clearance reduction leading to linezolid accumulation.29 Our results corroborate this recommendation, but we believe that further studies should be encouraged to determine the role of linezolid metabolites in linezolid-induced thrombocytopenia in patients with renal dysfunction, as well as to determine whether linezolid could be reduced by a dose adjustment according to renal function, for which there is currently no specific recommendation.
We observed no association of DPWD with the risk of thrombocytopenia, possibly because the mean body weight of the patients studied was equivalent to the recommended total daily dose of 1200mg linezolid and was below a defined cutoff (>20mg/kg/day). On the other hand, Niwa et al. observed a higher incidence of thrombocytopenia in patients receiving daily doses ≥22mg/kg.19 Natsumoto et al. also reported in their study that patients with DPWD ≥27mg/kg/day with body weight ≤45kg developed thrombocytopenia, suggesting the need for dose adjustment by body weight of adult patients, as already indicated for pediatric patients.7 Thus, in our opinion, the dose adjustment by body weight also in adults would be a good option in order to contribute to their safety, since this is a priority worldwide in hospitals.
With regard to other identified risk factors, we found that a low baseline platelet count (<200×103/mm3) was also considered a significant risk for thrombocytopenia in adults hospitalized in the Brazilian Midwest. In a retrospective cohort, evaluating factors associated with the development of linezolid-induced thrombocytopenia in adult Japanese patients, Niwa et al. reported an incidence of 44% (8/18) in patients with a low pre-treatment platelet count.19 Minson et al.30 identified that one variable independently associated with the development of thrombocytopenia (grade 3 and 4) was the presence of an initial blood platelet count between 50 and 99.9×103/mm, similar to the findings of Hanai et al.5
In conclusion, the incidence of linezolid-induced thrombocytopenia in adults hospitalized in the Brazilian Midwest was 18.2%. In addition, this study suggested that a platelet count ≤200×103/mm3, a level of serum creatinine >1.5mg/dL and renal impairment at the start of treatment can be considered risk factors for thrombocytopenia in adults. However, future prospective studies based on pharmacokinetics and pharmacodynamics are required to guarantee better strategies in the use of this antimicrobial. Nevertheless, we suggest the need for blood count monitoring during prolonged therapy (>7 days) to assess the occurrence of thrombocytopenia. These data may help multidisciplinary teams predict who is likely to develop thrombocytopenia during linezolid therapy and thereby reduce or avoid the risk of unnecessary damage in health care.
Availability of data and materialThe datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of interestThe authors declare no conflicts of interest.
Authors’ contributionsStudy conception and design: LSL and SSW. Acquisition of data: LSL, ECAB and KM. Analysis and interpretation of data: SSW, EBP and RTP. Drafting of manuscript: LSL, ECAB, KM and SSW. Critical revision: EBP and RTP.
The authors would like to thank the Universidade Federal de Mato Grosso do Sul (UFMS) for supporting the English language revision by EDITAL PROPP/UFMS 111/2018. ECAB and KM is thankful for the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) (Finance Code 001) for fellowships.