The thrombotic microangiopathies are diseases characterised by thrombocytopenia, microvascular thrombosis (intrarenal or systemic), erythrocyte fragmentation, elevated serum lactate dehydrogenase and microangiopathic hemolytic anemia. There are two main forms of the thrombotic microangiopathy (TMA): thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS).1 Moschcowitz first reported a case of TTP in 1924,2 while Gasser et al first described five cases of HUS in 1955.3 The differential diagnosis of TMA remains challenging, in particular in countries where many hospitals do not have routinely available activity assays, which delays diagnosis.4 Establishing the correct diagnosis is critical in ensuring the correct therapeutic intervention and avoiding patients being exposed to ineffective or toxic medications. We report the diagnostic journey of the patient presenting with TMA highlighting the diagnostic challenges.
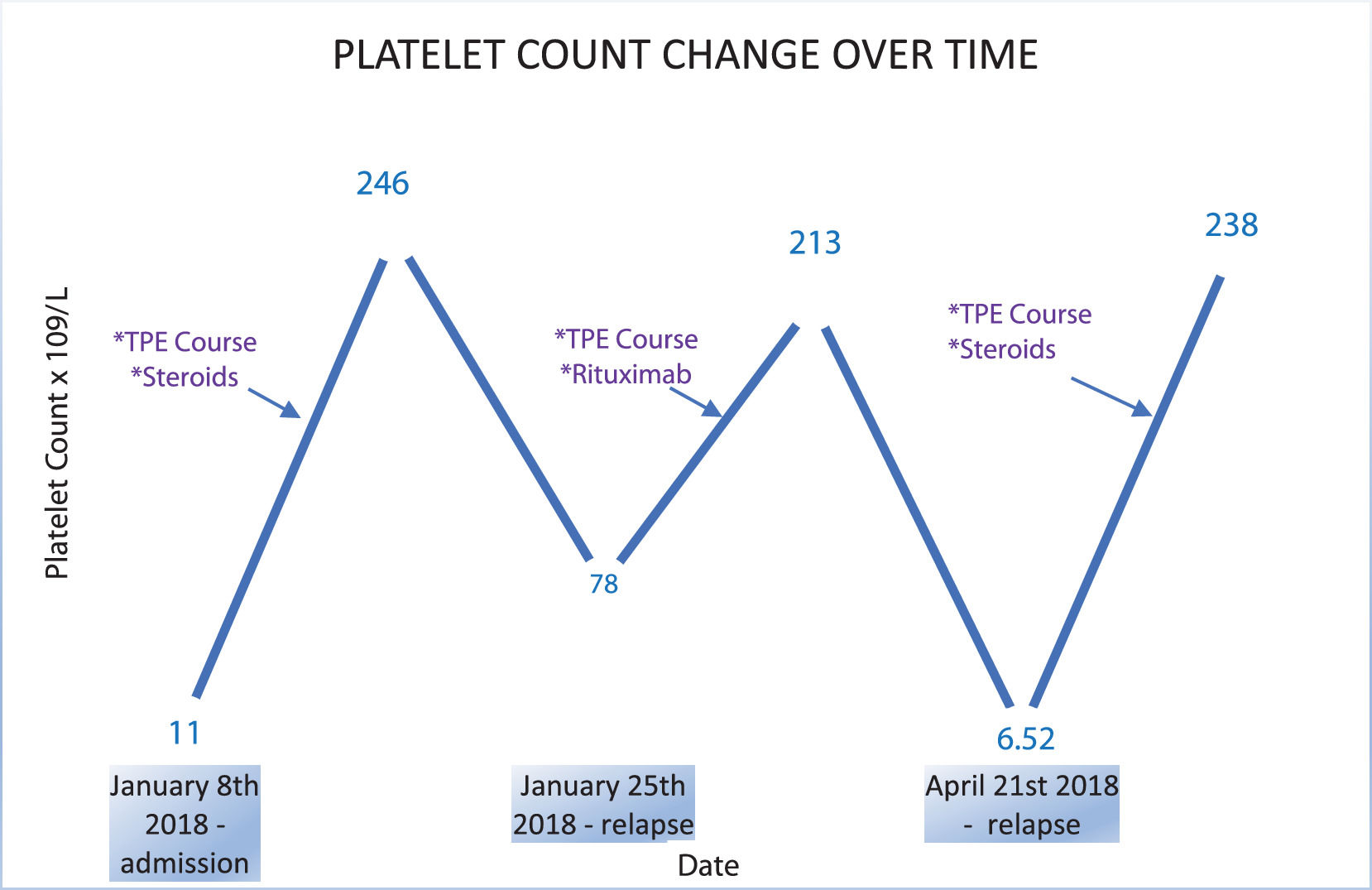
Case reportA 28-year-old male presented to his local hospital with nausea and rectal bleeding. Past medical history was unremarkable except of a history of thrombocytopenia 5 years prior to presentation. He had significant acute kidney injury (creatinine 1089 umol/l, (normal 45–115) requiring dialysis prior to being transferred to our center. Lab findings showed him to have evidence of haemolysis and thrombocytopenia (Figure 1.): WBC 10.36 × 109/L (normal range 4.00–10.0), Hgb 94 g/l (normal 138–175), platelets 11 × 109/L (normal 150–400), LDH 3619 U/L (normal 81–234), bilirubin 114.5 µmol/L (normal 1.7–20.5), peripheral smear showed: schistocytes; haptoglobin 1.8 g/L (normal 0.26–1.85), Coombs negative; Urine: proteinuria 2.0 g/l. A gastroscopy revealed massive bulbar erosions.
A diagnosis of thrombotic microangiopathy was made and the patient was started on therapeutic plasma exchange (TPE) with frozen plasma as a substitute. In total, four TPE procedures were performed and the patient was supported with hemodialysis (HD). The patient improved and kidney function, platelet count and hemoglobin normalized. In consultation with hematologists, corticosteroid therapy was initiated for what was assumed to be immune-mediated HUS. Two weeks later the patient relapsed with the platelet count falling to 78 × 109/L (normal 150–400), LDH elevated at 280 U/L (normal 81–234) and evidence of acute kidney injury with creatinine of 174 µmol/L (normal 45–115). Started on TPE again. After three TPE, lab results stabilized and platelets, LDH and kidney function normalised. Patient was started with Rituximab in the next four weeks, in dosage of 375 mg/m2 (once a week). The patient was discharged 6 weeks later with normal kidney function and no evidence of haemolysis. 43 days after discharge the patient was readmitted with a further relapse: Lab findings revealed low platelets 6.52 × 109/L (normal 150–400), an elevated LDH 1112 U/L (normal 81–234) and bilirubin 374 µmol/L (normal 1.7–20.5); complete blood count: RBC 3.40 × 109/L (normal 4.30–5.70), Hgb 109 g/L (normal 138–175), Hct 30.7% (normal 41.0–53.0), MCV 90.2 fL (normal 86–100). He was started on TPE again along with intravenous steroids but TPE had to be stopped as he developed a generalized urticarial reaction during the second treatment. After second TPE thrombocytes were normal, LDH was mildly raised (350–400 U/L – (normal 81–234). A week later he developed difficulty breathing and a CT pulmonary angiogram showed sub-segmental thromboembolism with thrombocytopenia (platelets = 86 × 109/L – (normal 150–400) and an elevated LDH 432 U/L (normal 81–234). Kidney function was normal and he was started on low molecular heparin. Afterwards, patient was clinically stable but closely monitored. Platelets were back to normal with plasma infusions every two weeks.
Diagnostic work up: Fortunately, we obtained blood samples from the patient on admission prior to his second course of TPE. This allowed for the first time appropriate diagnostic work up as outlined below:
ADAMTS13 activity and Antigen: In detecting ADAMTS13 activity and antigen we used TECHNOZYM® ADAMTS13 Activity and Antigen chromogenic ELISA Kits (Technoclone, Vienna, Austria). Pre-Plasma ADAMTS13 activity was <6% (normal range: 50–150%) showing significant deficiency of ADAMTS13 activity. For the functional inhibitor (Bethesda assay), we used a laboratory developed protocol based on ELISA activity. No ADAMTS13 inhibitors were detected, suggesting a diagnosis of congenital TTP. Genetic analysis and screening for mutations: We also performed the genetic analysis of CFH, MCP, C3, CFI, CFB, THBD, ADAMTS13, DGKE and C5 by Next generation Sequencing (NGS), in the DNA of this patient. The patient was found to be homozygous for a rare pathogenic variant in ADAMTS13, c.4143dupA, causing a frameshift and a premature protein interruption, (p.E1382SfsX6), previously associated with congenital TTP and severe ADAMTS13 deficiency.5 Notable, this patient has been also found to be heterozygous for a rare pathogenetic variant in MCP, c.475+1G>A, predicted to alter the splicing in exon 4 creating an alternative splice-site that results in the deletion of 21 nucleotides and a mutant protein that lacks 6 amino acids, previously reported in a patient with TMA and normal ADAMTS13 activity.6 In the above study, FACS analysis of MCP expression on granulocytes of the TMA patient heterozygous for the MCP c.475+1G>A variant showed a 50% reduction of MCP protein levels,6 indicating that the mutant protein is not expressed on cell surface.
Measurement of anti-FH antibody: The patient was negative for anti-Factor H antibodies.
Complement Assay: We have also tested the C5b-9 deposition levels induced ex vivo on resting and activated human microvascular endothelial cells (HMEC-1) 7 by the serum of this patient. The C5b-9 deposits were 249% on resting and 266% on activated HMEC cells (normal value <150%). Thus, the serum of this patient caused increased C5b-9 deposition levels, consistent with an activation of the terminal complement pathway.
DiscussionTTP and HUS are phenotypic manifestations of TMA. HUS may manifest as: (a) Typical HUS, associated with infection by strains of E.coli bacteria that produce shiga-like toxins; (b) Atypical HUS, associated with genetic abnormalities affecting regulators and components of the complement alternative pathway, including complement factor H (CFH), membrane cofactor protein (MCP), complement factor I (CFI), C3, complement factor B (CFB), and thrombomodulin (THBD), or with anti-CFH autoantibodies, combined with trigger such as infection, drug exposure or pregnancy.1 It is more common in children and young adults and the prognosis is poor with high morbidity and mortality in acute phase; and c) Secondary HUS that may occur as a complication of autoimmune diseases, pregnancy, malignant hypertension and transplantation.1 TTP is caused by deficiency of the von Willebrand cleaving protease ADAMTS13, which was first identified by Furlan et al.8,9 Two mechanisms for deficiency of the ADAMTS13 activity have been identified, an acquired deficiency due to the formation of anti-ADAMTS13 autoantibodies (acquired TTP) and a genetic deficiency due to homozygous or compound heterozygous mutations in ADAMTS13 (congenital TTP). In our patient, very low ADAMTS13 activity (<6%) in the absence of ADAMTS13 inhibitors, was suggestive of congenital TTP,10 which can be under diagnosed. The finding of elevated serum-induced C5b-9 deposits on cultured endothelial cells in our patient ex-vivo, is consistent with published data showing that the alternative pathway of complement is abnormally activated in patients with congenital TTP, likely due to uncleaved ultra-large VWF multimers acting as a platform for C3 activation on endothelial cell surface.11 The concomitant presence of the heterozygous pathogenetic variant in MCP could act as a genetic modifier and contribute to complement dysregulation in vivo and to the renal dysfunction in this patient. This interpretation is supported by previous published reports of complement gene abnormalities in congenital TTP patients with renal insufficiency but not in patients without renal involvement.12 Published reports suggest that congenital TTP can present later during puberty, pregnancy, with precipitating factors, and understanding and possibly avoiding these triggering factors is very critical in preventing relapse.13 Our patient with congenital TTP had a good therapeutic response to TPE with fresh frozen plasma.14
ConclusionCongenital TTP is very rare TMA. Some but not all congenital TTP patients manifest with renal insufficiency with mutations in complement regulatory genes. Taken together, this case is the first report of a patient with congenital TTP who carries a rare pathogenic MCP mutation. Congenital TTP can often be under diagnosed, suggesting the importance of diagnostic assays including complement genotyping and ADAMTS13 activity in patients presenting with TMA, to avoid unnecessary treatment.
The authors wish to thank Drs. Marina Noris, and Paola Cuccarolo and Sara Gastoldi (Istituto di Ricerche Farmacologiche Mario Negri IRCSS, Bergamo Italy) for the identification of the ADAMTS13 mutation and for the assay of serum induced C5b-9 deposition on HMEC-1.