Inflammation and angiogenesis are linked to the development of cancer since both can support the establishment of a tumor-prone microenvironment. The CCR5 is a major regulatory molecule involved in inflammation. The CD34 molecule is commonly described as a hematopoietic stem cell marker, and CD34+ cells are involved in the regulation of distinct physiological processes, including angiogenesis. CCR5 participates in the development of various types of cancer, and recently, a reduced CCR5 expression was associated with low CD34+ cell counts in human cord blood. A naturally occurring genetic variant of the CCR5 gene, the so-called CCR5Δ32 polymorphism, consists of a 32 base-pair deletion in the DNA, interfering in the CCR5 protein levels on the cell surface. When in homozygosis, this variant leads to a total absence of CCR5 expression on the cell surface. In heterozygous individuals, CCR5 surface levels are reduced. Based on these key findings, we hypothesize that a functional interaction can connect CCR5 and CD34 molecules (giving rise to a “CCR5-CD34 axis”). According to this, a CCR5-CD34 interaction can potentially support the development of different types of cancer. Consequently, the lack of CCR5 in association with reduced CD34+ cell counts could indicate a protective factor against the development of cancer. It is required to characterize in detail the functional relationship between CCR5 and CD34 proteins, as well as the real influence of both molecules on the susceptibility and development of cancer at population level. If our hypothesis is confirmed, the CCR5-CD34 axis may be a potential target in the development of anti-cancer therapies.
The CD34 molecule is a commonly targeted antigenic determinant used as a characteristic marker to isolate and analyze hematopoietic stem cells.1–9 This protein is expressed by all hematopoietic stem cells and its expression is lost during the cell differentiation. Therefore, mature hematopoietic cells are usually CD34 negative.1 On the other hand, this molecule is also present in a variety of mature cell types, as mesenchymal cells, muscle satellite cells, corneal keratocytes, interstitial cells, epithelial progenitors, vascular endothelial progenitors, and activated endothelial cells.10 Of note, CD34 expression has been already correlated to vasculogenic and angiogenic processes, the mesenchymal CD34+ stem cells being closely associated to vascularization.1,4–6,8,9,11 Importantly, both vasculogenic and angiogenic processes are essential to the development of cancer.12–17Additionally, there are numerous studies describing CD34 expression in tumor cells,2,5,6,9,18 although the loss of this molecule has been associated with an invasive malignant phenotype.8,19 In this context, the CD34 molecule can be viewed in neoplastic cells as a dedifferentiation marker. Also, CD34 could be expressed by endothelial cells and endothelial progenitors as a potential tumor vascularization marker.
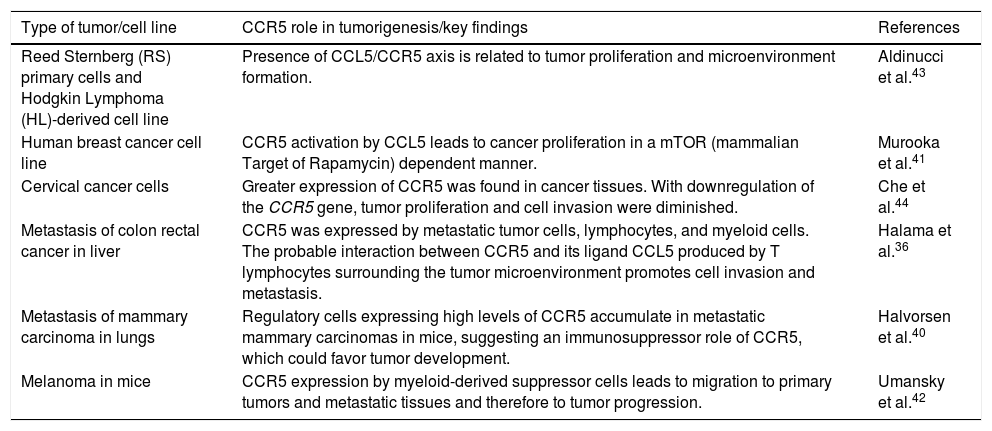
CCR5: a chemokine receptor with multiple inflammatory functionsChemokines are fundamental regulators of the development, differentiation, and migration of leukocytes.20–24 In addition, chemokines participate in the angiogenic process.21,25 The CC Chemokine Receptor 5 (CCR5) is a well-known example of such molecules, being especially involved in leukocyte migration.26 Furthermore, CCR5 is well-known due to its classic role as a co-receptor molecule used by the HIV type I viruses.21,25,27–31 In addition, CCR5 acts in Th1 immune responses and the lack of signaling through this molecule leads to a shift in the Th2 responses.20,21 Several chemokines were described as efficient agonists of this receptor, such as RANTES (CCL5), MIP-1α (CCL3) and MIP-1β (CCL4). On the other hand, some chemokines interact less efficiently with CCR520–22,25,27,32 or are classified as CCR5 antagonists, which is the case of CCL7.25,29 Of note, the impact of CCR5 on the development of cancer is an emerging topic, with several studies suggesting that CCR5 plays an important role in the establishment of the tumor microenvironment and progression of different types of cancer. Notably, the CCR5 molecule has been related to the migration and spread of tumor cells, and therefore, to metastasis. Moreover, the presence of the receptor in neoplasms has been associated with the migration of regulatory cells and generation of an immunosuppressor tumor-prone environment. Detailed examples and other effects of CCR5 on tumorigenesis are shown in Table 1.22–24,33–44
Roles of CCR5 in tumorigenesis.
| Type of tumor/cell line | CCR5 role in tumorigenesis/key findings | References |
|---|---|---|
| Reed Sternberg (RS) primary cells and Hodgkin Lymphoma (HL)-derived cell line | Presence of CCL5/CCR5 axis is related to tumor proliferation and microenvironment formation. | Aldinucci et al.43 |
| Human breast cancer cell line | CCR5 activation by CCL5 leads to cancer proliferation in a mTOR (mammalian Target of Rapamycin) dependent manner. | Murooka et al.41 |
| Cervical cancer cells | Greater expression of CCR5 was found in cancer tissues. With downregulation of the CCR5 gene, tumor proliferation and cell invasion were diminished. | Che et al.44 |
| Metastasis of colon rectal cancer in liver | CCR5 was expressed by metastatic tumor cells, lymphocytes, and myeloid cells. The probable interaction between CCR5 and its ligand CCL5 produced by T lymphocytes surrounding the tumor microenvironment promotes cell invasion and metastasis. | Halama et al.36 |
| Metastasis of mammary carcinoma in lungs | Regulatory cells expressing high levels of CCR5 accumulate in metastatic mammary carcinomas in mice, suggesting an immunosuppressor role of CCR5, which could favor tumor development. | Halvorsen et al.40 |
| Melanoma in mice | CCR5 expression by myeloid-derived suppressor cells leads to migration to primary tumors and metastatic tissues and therefore to tumor progression. | Umansky et al.42 |
As mentioned above, the CCR5 protein has a regulatory effect on inflammatory cells,26,27 thus it must have an enhancing function in the migration of pivotal cells for tumorigenesis, explaining at least partially its connections with the development of cancer. Importantly, the CCR5 expression was already observed in some regulatory T (Treg) cell subsets.45–50 In certain circumstances and types of cancer, Treg cells were linked with a better prognosis.51 Nevertheless, we are facing a two-edged sword, since Treg cells can also be subverted by tumor cells in order to generate a tolerogenic environment, allowing the proliferation and establishment of neoplasms.52–55 Thus, we can suppose that the absence of CCR5 in Treg cells may be a protective factor in the context of tumorigenesis.
Inflammation and the development of cancerThe role of the immune system in the development of cancer is a field of extensive debate. Even though inflammatory cells could eliminate tumor cells, inducing their death, they are also important components of the tumor microenvironment, sometimes favoring tumor growth and proliferation, also promoting neoangiogenesis.36–38 In this sense, some immune cell-derived factors, when in disbalance, can promote tumor progression. Adding to the complexity of the interactions happening in the tumor, there is a wide variety of immune cells in a neoplastic environment, including lymphocytes, macrophages, neutrophils and dendritic cells, which can produce cytokines and other mediators that enhance the tumor development.56 The immune surveillance and the individual responses to cancer therapy are also affected by different inflammatory patterns.57
CCRΔ32 and the CD34+ stem cell repertoire in the cord blood - a puzzling observationRecently, a study performed by Enrich et al.58 raised intriguing results. A cohort of Spanish cord blood donors was genotyped for the CCR5Δ32 polymorphism (rs333), a genetic variant which consists of a 32 base-pair deletion in the open reading frame of the CCR5 gene. This deletion causes a premature stop-codon, which leads to the formation of a truncated protein that is not expressed on the cell surface.30,31 Heterozygous and homozygous individuals for CCR5Δ32 show respectively reduced CCR5 expression and no expression of the CCR5 molecule on the cell surface.59,60 As previously mentioned, CCR5 is an HIV-I co-receptor and this genetic variant became widely known due to its association, when in homozygous, to resistance against HIV infection.61,62 The main objective of Enrich et al.58 was to identify the CCR5Δ32 homozygous cord blood units, which could be used as donor cells to be transplanted to HIV+individuals. The idea was that the CCR5Δ32 homozygous cord blood units would repopulate the host with a set of cells which would not allow HIV infection, avoiding the maintenance of the virus infection, eventually leading to viral clearance. A similar approach using bone marrow CCR5Δ32 homozygous cells has been successfully applied in one patient, who remains with sustained suppression of the HIV infection.63–65 Nevertheless, among the results of Enrich et al.,58 one was surprising and unprecedented: a smaller amount of CD34+ cells was found in the samples from the CCR5Δ32 homozygous donors, as compared to both heterozygous and CCR5 wild-type homozygous. This situation represents a drawback to the main objective of the authors since a) fewer cells would be available from these donors and b) potentially cord blood units from those CCR5Δ32 homozygous donors would present a lower reconstitution potential of the host leukocyte repertoires. In this same study,58 Enrich et al. suggested that CCR5 and its agonist MIP-1α (CCL3) play a critical role in the hematopoietic stem cell function. Specifically, MIP-1α possibly regulates cytokine-induced stem-cell proliferation and the lack of CCR5 in Δ32/Δ32 individuals might disrupt the MIP-1α signaling pathway and explain the lower CD34+ cell counts.20,58,66–68 From this unexpected result, a new point emerged: a connection between CCR5 and CD34+ cells.
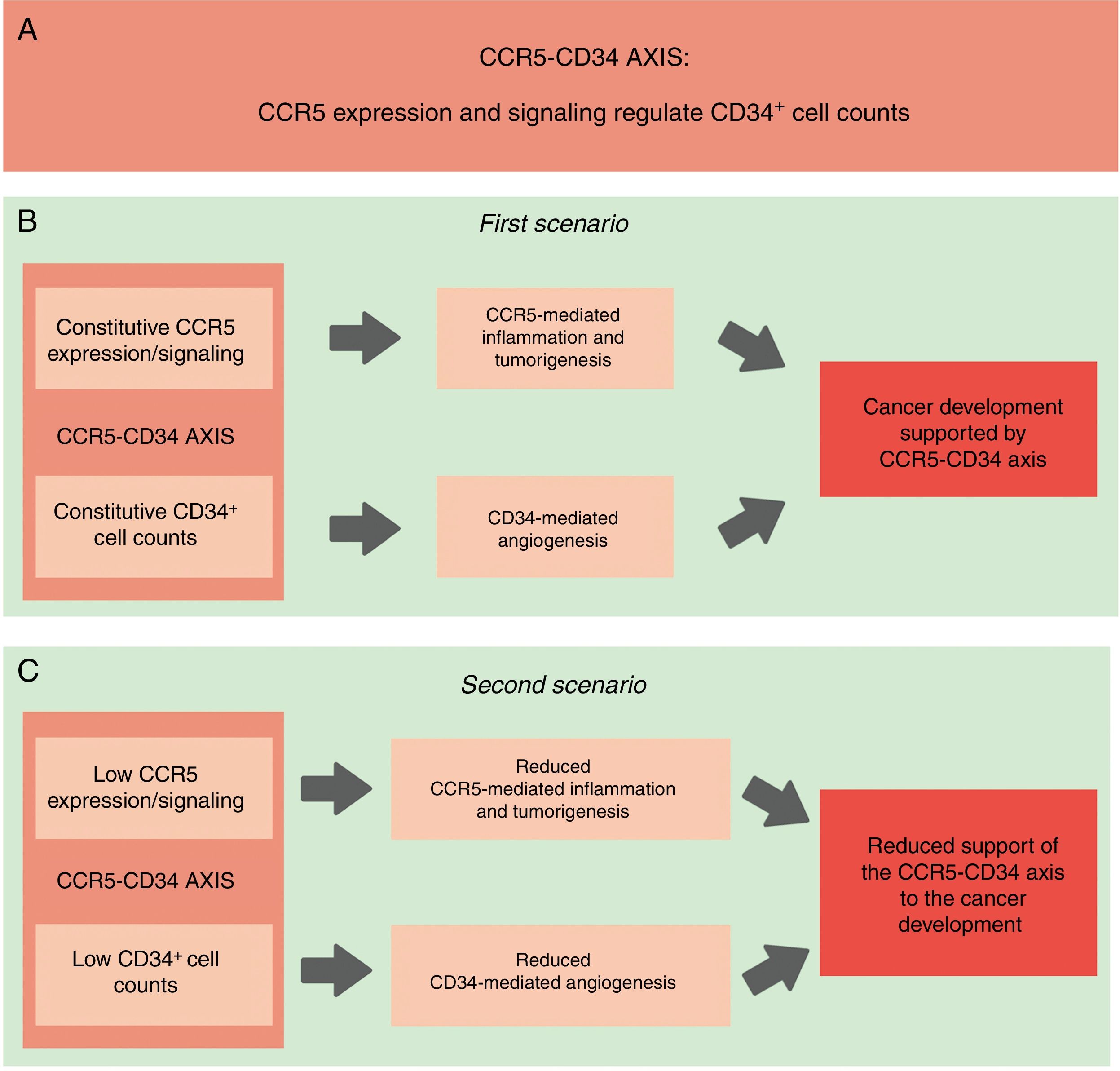
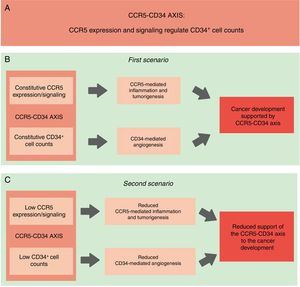
Linking CD34+ cells, the CCR5+ repertoire and cancer - the hypothesisConsidering that CD34+ cells were already associated with tumor development, and taking into account that the absence of CCR5+ cells (which happens in homozygous individuals of the CCR5Δ32 variant) is linked with a lower proportion of CD34+ stem cells, it is possible to infer that a functional interaction between CCR5+ and CD34+ cells exists and that this interaction can give support to cell proliferation and expansion and perhaps, to the establishment/development of different types of cancer. Consequently, the reduced expression of CCR5, in association with low CD34+ cell counts, would protect against the establishment of a tumor microenvironment and the development of cancer (Fig. 1). Additionally, data from previous studies show that the CCR5 agonist MIP-1α has a direct effect on CD34+ cells,32,58,67,68 suggesting a functional connection between CCR5 and CD34. Thereafter, this functional correlation between CCR5 and the CD34+ cells will be referred to as the “CCR5-CD34 axis”. Importantly, our hypothesis mainly addresses the role of CCR5 in the migration of inflammatory cells to the tumor environment (CCR5 as a mediator of inflammation). However, as previously mentioned, CCR5 and its ligands play an important role in Treg cell recruitment and migration of tumor cells. These different roles of CCR5 in tumorigenesis needed to be considered in our hypothesis. Taken together, an association between CCR5 and CD34 may support tumorigenesis.
Schematic representation of our hypothesis. (A) CCR5-CD34 axis: functional interactions between CCR5 and CD34. (B) CCR5-CD34 axis supports the development of different types of cancer. Constitutive: normal/expected levels of CCR5 and CD34+ cells. (C) Reduced CCR5 expression in association with lower CD34+ cell counts would protect against the establishment of the tumor microenvironment and the development of cancer.
The unravelling of the mechanisms of tumorigenesis is crucial for the development of new drugs and effective treatments against different types of cancer. Here we hypothesize on the existence of a not yet described interaction between two important molecules of the immune system that may act together as tumorigenesis promoters. Potentially, the CCR5-CD34 axis could be a new target for cancer treatments, pharmacologically, or as part of gene therapy strategies. However, prior to this, the interactions between CCR5 and CD34 need to be characterized at both cellular and functional levels, and their real importance in the tumor biology needs to be understood in detail.
In general, the lack of CCR5 observed in homozygous individuals of the CCR5Δ32 variant is considered not to be associated with any severe essential physiological alterations. Although it is generally accepted that homozygous individuals of this variant have no severe immunological or clinical deficiencies,30,69 exacerbated inflammatory responses have already been reported in association with the CCR5Δ32 variant.70 Interestingly, a recent report has linked CCR5 absence with defective bone development.71 Thus, it is crucial to consider that CCR5 mediates different immune functions and it is possible that its absence promotes changes of difficult detection, but with medical significance in a specific environment or context. For example, homozygous individuals of CCR5Δ32 are more susceptible to develop symptomatic West Nilo virus infection.72,73 Nevertheless, CCR5Δ32 is a pleiotropic variant, promoting different outcomes in different situations.74 Of note, especially considering the recent alleged report of CCR5 editing in humans, the pros and cons of the absence of CCR5 were addressed in a recent publication by our group.75 If our hypothesis is confirmed, the physiological significance of the absence of CCR5 needs to be reviewed. Although the chemokine-ligand system is robust and redundant,76 which ensures its functioning in the absence of some specific chemokine or chemokine receptor, alterations in such a balanced system may have a significant impact on other diseases (in a favorable or unfavorable way). Looking at the hypothesis described here, the lack of CCR5 could be considered a protective factor against the development of cancer.
Testing our hypothesisWe suggest the following initiatives to test our hypothesis:
- -
First, it is necessary to investigate the CD34+ cell counts in individuals with different CCR5Δ32 genotypes in different populations with the aim of confirming the correlation between the CCR5 expression and CD34+ cell counts.
- -
Second, functional studies must be performed to evaluate the potential interactions between CCR5 and CD34+ cells, helping to understand the biological significance of the CCR5-CD34 axis and identify in which contexts such interactions occur.
- -
Third, it is necessary to evaluate the influence of the lack of CCR5 and the reduced CD34+ cell counts (separately and in association) on the development of cancer at a populational level, as well as in in vitro strategies.
- -
Fourth, it would be interesting to evaluate tumor growth in CCR5 and CD34 knockout animals and controls, aiming to compare the tumor progression between the groups.
- -
Finally, we suggest investigating the frequency of CCR5Δ32 and the cancer incidence at the populational level (in different geographic regions), in order to verify the correlation between these two data sets.
Each cancer type comprises specific cellular and molecular environments. However, migration of inflammatory cells to the tumor site and angiogenesis are processes found in different types of solid tumors. Thus, if the influence of the CCR5-CD34 axis on the development of cancer is confirmed, it is possible that both molecules become targets for the development of new therapeutics against tumors. In addition, pharmacological strategies focusing on modulating both molecules together may be quite promising. Currently, the use of CCR5 blockers is already being suggested for the treatment of different pathologies, besides HIV infection, including cancer.23,24,36–38,77 The above-mentioned scenario shows that our hypothesis deserves to be investigated, once (I) it will contribute to the understanding of the factors that support the establishment of the tumor microenvironment and modify the susceptibility to the development of cancer and (II) it has the potential to reveal important implications for cancer treatment.
FundingBKL receives a scholarship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil). JHE received a doctoral scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil). Currently, JHE receives a postdoctoral fellowship from CAPES (Brazil). JABC receives a research fellowship from CNPq (Brazil).
Meeting of ethical standardsWe declare that this study was developed according to all rules and meeting all ethical standards.
Conflicts of interestThe authors declare no conflicts of interest.