Coronavirus disease 2019 (COVID-19), an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported as a series of pneumonia cases in China.1 Since then, due to the high transmissibility of SARS-CoV-2, the disease has spread worldwide, causing a pandemic that has affected more than 521 million people.2 The vaccines currently available against COVID-19 show an excellent efficacy and safety profile.3 Clinical evidence has indicated that vaccination against COVID-19 protects against severe disease symptoms but is also an important tool to reduce the spread of the virus and the rate of infection. Vaccinated individuals have a lower risk of transmitting the virus; however, it is crucial to continue to practice responsible behavior.4,5
Originally thought to be a respiratory pathogen, SARS-CoV-2 can lead to multiple organ dysfunction and cause a variety of complications (e.g., gastrointestinal, neurologic, hematologic, thromboembolic, immunologic, and cardiovascular complications, for example).6 Many of the severe complications of COVID-19 are related to hypercoagulable conditions, such as thrombotic microangiopathies.7
Thrombotic microangiopathy (TMA) is a broad term that encompasses both a pathologic (microvascular or macrovascular occlusive disease, often with intraluminal thrombus formation) and a clinical definition (microangiopathic hemolytic anemia (MAHA) with thrombocytopenia).8 A common phenotypic manifestation of TMA is thrombotic thrombocytopenic purpura (TTP), a medical emergency characterized by MAHA, severe thrombocytopenia, and ischemic end-organ damage due to microvascular platelet-rich thrombi.8 Here, we report the case of a patient admitted to the hematology department of a public children's hospital in Belo Horizonte, Brazil, for evaluation of acquired TTP due to SARS-CoV-2 infection. We also found the patient to have marked poikilocytosis with numerous schizocytes and mushroom-shaped erythrocytes (or pincer cells).
Case reportA previously healthy 14-year-old male patient presented to a health care facility on February 4, 2021, with paralysis, speech disturbances, headache, and an isolated episode of fever. Laboratory tests revealed anemia (hemoglobin: 7.2 g/dL) and thrombocytopenia (35,000/mm³). A positive epidemiology for COVID-19 was reported, with his sister becoming symptomatic the same day the patient's symptoms began. Subsequently, his sister's SARS-CoV-2 infection was confirmed by reverse transcriptase polymerase chain reaction (RT-PCR), which was negative in the patient.
Four days after the initial symptoms, the patient developed generalized tonic-clonic seizures accompanied by mental confusion and somnolence (ECG10–12). In addition, he had self-limited gingival bleeding, macroscopic hematuria, worsening anemia, and thrombocytopenia. As a result, he was referred to us and admitted in a febrile state (axillary temperature of 39 °C).
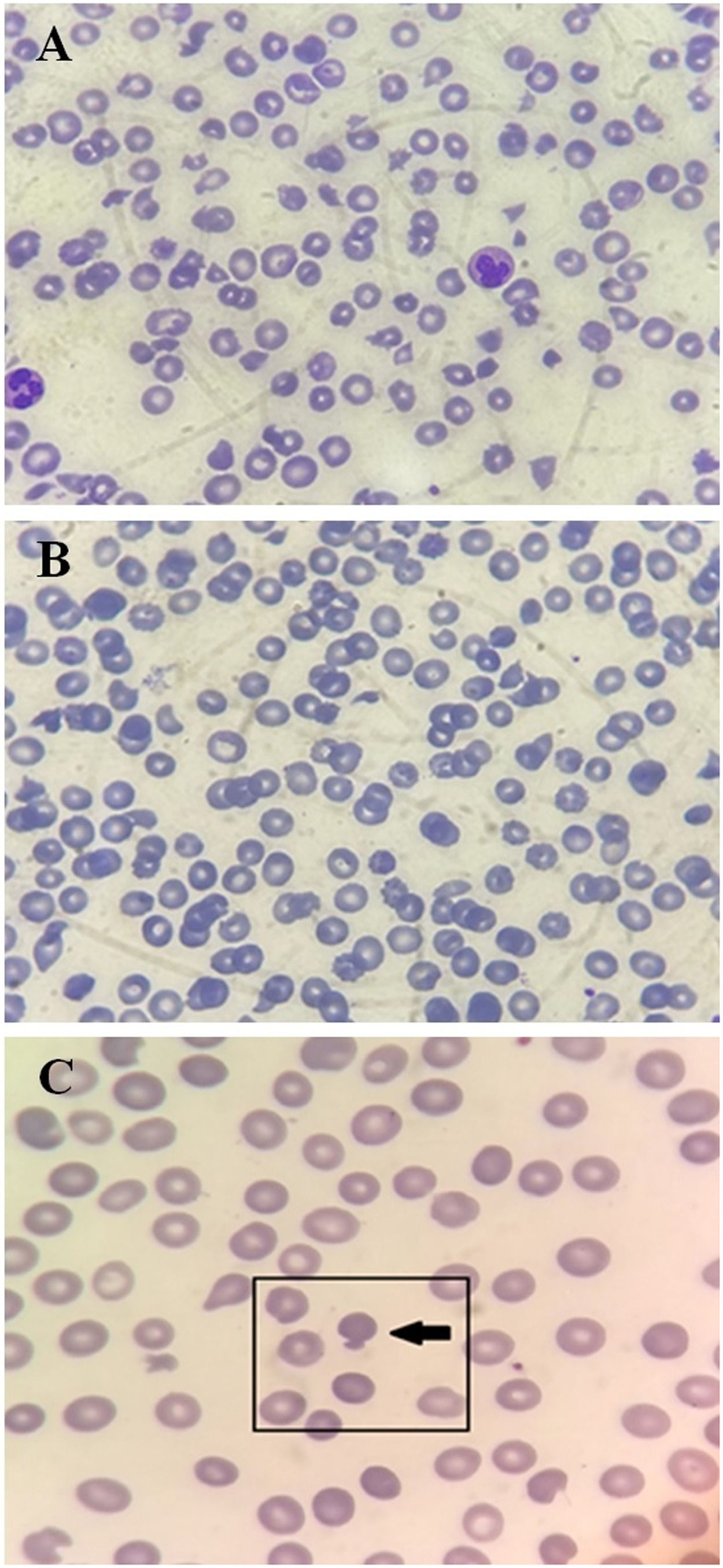
Because of the hematologic changes, he received a transfusion of 5 platelet units randomly at his home hospital without any increase in platelet count or clinical improvement. Further examination revealed hemolytic anemia, decreased haptoglobin (1 mg/dL), a negative direct Coombs test, and a peripheral blood smear with numerous schizocytes and mushroom-shaped erythrocytes (pincer cells) (Figure 1). On the 10th day after the onset of symptoms, his laboratory values worsened (platelets of 16,000 per mm3) and he received a transfusion of 600 ml of fresh frozen plasma, which did not respond. The following day, the platelet count was 13,000/mm³. In addition to the critical platelet levels, there was a drop in hemoglobin. He then received 300 ml of packed red blood cells and 5 days of methylprednisolone pulse therapy on day 12.
Photomicrograph of a panoptically stained blood smear from the patient at the time of imaging (A) showing severe microcytosis, hypochromia, polychromasia, schizocytes, and mushroom-shaped red cells; and after clinical remission (B). Mushroom-shaped cells are also highlighted in (C). All images are magnified 1000 times.
On February 18, 2021, he underwent magnetic resonance angiography (MRA) of the brain, which showed a small focus with diffusion restriction in the topography of the left semioval center, probably related to a recent ischemic vascular insult (focus of ACVi in the subacute phase), large arterial trunks that were permeable intracerebrally, with smooth and regular contours, without signs of aneurysms, malformations, or significant stenoses. Based on these findings, a comprehensive etiologic workup was performed, including rheumatologic studies, myelogram, echocardiogram, and blood cultures, all of which were without changes. A CSF examination was not performed because of significant thrombocytopenia. Given the adolescent's clinical and laboratory features (i.e., thrombocytopenia, microangiopathic hemolytic anemia, neurologic changes, and hematuria without renal or hepatic dysfunction), a diagnosis of acquired thrombotic thrombocytopenic purpura (TTP) was considered. ADAMTS13 test was not performed due to technical difficulties. However, the PLASMIC score was six, meaning the patient was at high risk of severe ADAMTS13 deficiency.9
The patient showed no improvement in laboratory values, and on day 20 after the onset of symptoms, he still had significant thrombocytopenia (13,000/mm³), but it was clinically stable. In this context, a referral for plasmapheresis was requested and another COVID-19 test was performed. On March 5, 2021, RT-PCR was positive for SARS-CoV-2. While waiting for plasmapheresis, the patient experienced a spontaneous and unremarkable improvement in laboratory values (hemoglobin of 8.9 g/dL and platelets of 39,000/mm3, with a decrease in schizocyte count). In the following days, clinical and laboratory improvement occurred (blood count with only 2.5% schizocytes), without criteria for plasmapheresis. The procedure was then discontinued, and the patient was discharged on March 10, 2021. Fourteen days after discharge, at an outpatient consultation, he already had a complete improvement of the hemogram (hemoglobin of 12.6 g/dL and platelets of 313,000/mm3) and remained asymptomatic.
DiscussionTTP is caused by a severe deficiency of the plasma metalloproteinase ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type 1 repeats, member 13), the key enzyme involved in the cleavage of very large von Willebrand factor (VWF) multimers into smaller, less procoagulant multimers.8,10 This coagulopathy can be either congenital, due to mutations in the ADAMTS13 gene, or acquired, due to the formation of autoantibodies or a decrease in serum ADAMTS13 levels. Many factors can trigger acquired TTP, including inflammatory diseases, medications, and pregnancy after allogeneic hematopoietic stem cell transplantation.8,11 In addition, acute infections are a well-known, well-established trigger for acquired TTP.10
In this case, we present a 14-year-old male patient with TTP associated with COVID-19. Similar reports were identified in previous studies. Nicolotti et al.8 showed a 44-year-old woman admitted to the emergency department with severe anemia, acute renal impairment, and COVID-19 respiratory failure. Clinical and laboratory findings suggested thrombotic microangiopathy. Considering the temporal sequence and the absence of other possible causes, SARS-CoV-2 infection may have been the precipitating factor for the development of TTP. In another case, a 35-year-old woman was diagnosed with positive COVID-19 and associated TTP after admission with right-sided weakness and slurred speech.12 In both cases, ADAMTS13 activity was absent or reduced in the presence of an inhibitor. Furthermore, Capecchi et al.10 reported a 55-year-old woman with a previous episode of TTP due to bacterial pneumonia 30 years ago, successfully treated at that time with corticosteroids and plasmapheresis, who suffered a relapse of TTP triggered by the SARS-CoV-2 virus in late March 2020, during the COVID-19 pandemic in Italy.13 Another patient, a 14-year-old girl, was admitted to a health center in the Brazilian state of São Paulo in August 2020 with headaches and altered consciousness. Previously, the patient presented fever (body temperature 38 °C), cough, dyspnea, fatigue, and malaise for about 11 days, which resolved spontaneously within about 20 days. All examinations were consistent with acquired TTP, and serologic testing for COVID-19 showed a positive IgG fraction.14
The exact pathologic mechanism leading to TTP after SARS-CoV-2 infection is not fully understood. However, possible causes include direct endothelial damage, a high inflammatory state associated with a cytokine storm, and/or increased procoagulant factors such as factor VIII, VWF, and fibrinogen.15 Pascreau et al.16 have shown that patients with COVID-19 have a marked increase in VWF levels and an associated moderate deficiency in ADAMTS13, which are risk factors for TTP. In vitro studies have shown that SARS-CoV-2 spike protein 1 leads to the release of abnormally large VWF from endothelial storage sites.17 This partially traps ADAMTS13 on the endothelial surface, where it cleaves nascent VWF on the endothelial surface, resulting in a mild to moderate decrease in circulating ADAMTS13.16,17 In addition, alterations in level of consciousness, fever, and thrombocytopenia are findings of TTP frequently observed in COVID-19 cases, suggesting a pathophysiological overlap of these two clinical conditions.13
Rapid recognition of TTP in patients with COVID-19 is crucial for the development of appropriate therapy and prevention of sequelae.16 The first line of treatment is based on daily therapeutic plasmapheresis with or without corticosteroid therapy (mainly methylprednisolone or dexamethasone). In addition, modulators of the immune system may also be included in treatment.8,10 For example, Darnahal et al.17 have shown that treatment of TTP associated with COVID-19 with the immunosuppressant rituximab (375 mg/m2, weekly for four consecutive weeks) is effective. Due to the prolonged onset of action of rituximab, plasma exchange must be continued until the drug takes effect.18 In turn, Singh et al.11 showed that the use of caplacizumab (10 mg for 4 weeks), a humanized single variable domain immunoglobulin (nanobody) against VWF, resulted in complete clinical remission of TTP in an adult female patient (44 years) with COVID-19.
Hematologic abnormalities such as poikilocytosis, anemia, and thrombocytopenia may occur in COVID-19 patients.11 Erythrocyte morphology in the peripheral blood smear of the patient who participated in this study showed some mushroom-shaped red cells in addition to numerous schizocytes. This finding has been previously reported in the literature. Following observation of red blood cell morphology in patients with COVID-19, a cohort that systematically reviewed the blood films of 50 individuals infected with SARS-CoV-2 showed that 66% of patients (33 patients) had mushroom-shaped red cells.18 These findings suggest that SARS-Cov-2 infection can significantly affect red blood cell physiology. Mushroom-shaped red blood cells are observed primarily in oxidatively induced hemolysis due to removal of two Heinz bodies.18 Their presence in patients with SARS-CoV-2 infection therefore suggests a possible role of oxidative stress in the pathophysiology of the disease.19
ConclusionThe exact pathophysiology of the association between TTP and COVID-19 is not fully understood and may be related to endothelial damage, cytokine storms, or inhibition of ADAMTS13. Because of the pandemic potential of SARS-CoV-2 and its associated symptoms, it is essential to evaluate the hematologic parameters of patients with moderate and/or severe disease, taking into account the occurrence of PTT, in order to initiate appropriate and more efficient treatment as early as possible and to avoid complications and sequelae. Although a direct causal relationship between COVID-19 and TTP is difficult, clinicians should have a high index of suspicion for TTP in an appropriate clinical scenario with relevant laboratory features.
Sources of support in the form of grantsNone.
Ethical approvalCEP (FHEMIG) - 59488422.3.0000.5119
All procedures performed in studies involving human participants conformed to the ethical standards of the institutional and/or national research committee and the 1964 Declaration of Helsinki and its subsequent amendments or comparable ethical standards.