Thrombotic thrombocytopenic purpura (TTP) is a thrombotic microangiopathy (TMA), marked by a severe deficiency of ADAMTS13 (disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) and is characterized by microangiopathic hemolytic anemia (MAHA), thrombocytopenia and organ ischaemia.1 In patients with congenital TTP (cTTP), ADAMTS13 deficiency is secondary to biallelic mutations in the ADAMTS13 gene, whereas acquired TTP is immune-mediated (iTTP), characterized by the presence of autoantibodies against ADAMTS13.2 The recognition of the disease and initiation of therapeutic plasma exchange (PEX) is the mainstay of the treatment. TTP is a rare disease, with an estimated incidence of 2.17 per million per year.3 It is more frequent in women, and the median age of onset is 43 years.2 iTTP has been related to predisposing conditions in a subset of patients, such as autoimmune disease, infection and human immunodeficiency virus (HIV), cancer, drugs, and, also, related to pregnancy itself. However, most cases (59%) occurs without any predisposing factor, being described as primary immune-mediated.4
Thrombocytopenia is a prevalent finding during pregnancy, and it is more often related with gestational thrombocytopenia, preeclampsia or HELLP syndrome, immune thrombocytopenia, rheumatologic diseases or disseminated intravascular coagulation. TTP is a rare cause in this setting, although remarkable for its severity. Hence, the diagnostic approach is challenging and distinguishing these disorders is essential to decrease the maternal-fetal morbidity and mortality. We report a 29 years old female patient with a TMA related to HIV and pregnancy, who was successfully treated with PEX. The patient gave written consent for publication.
Case reportA 29-year-old pregnant woman during her second trimester came to the emergency department with a history of diffuse bruises and petechiae, associated with epistaxis, asthenia and moderate holocranial headache over the previous week. In the previous 24 hours she also has started with a sudden paraesthesia in her left upper limb and left facial side. She had no motor deficit and no other symptoms. From her past medical history, she had been diagnosed with gestational diabetes in her first pregnancy and underwent a cesarean delivery with 40 weeks of gestational age due to failed induction of labor and non-reassuring fetal condition. She was obese and had no medication use history. She had a history of sexual intercourse with multiple partners and irregular preservative use. Her previous serologies tests were made during her first pregnancy, 7 years before. She arrived with a gestational age of 27 weeks and 6 days based on a fetal ultrasound of 25 weeks and 5 days. She had a history of incomplete prenatal care with only one appointment during the whole gestation. Thus, she had no screening tests from prenatal care.
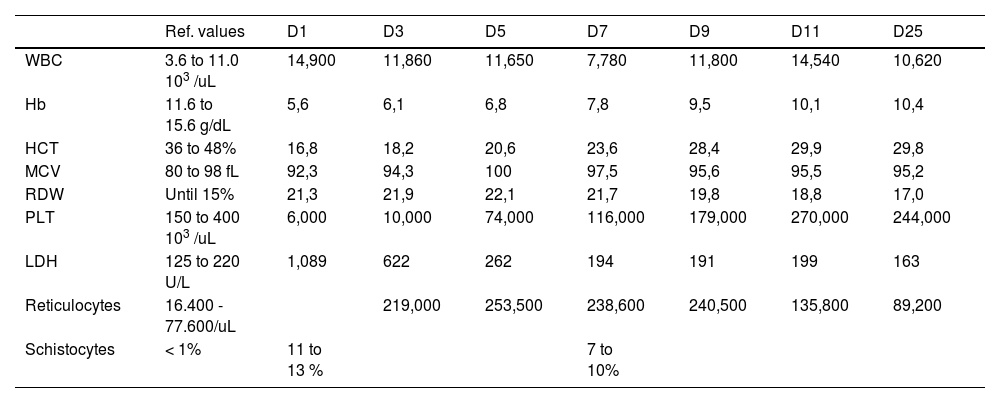
On examination, she had mild tachycardia (107 beats per minute), normal blood pressure (103/57 mmHg), petechiae and ecchymosis throughout her body and normal neurological assessment. Obstetrical evaluation revealed absent uterine dynamics, present fetal movement, fetal heartbeat of 130 beats per minute, uterine height compatible with gestational age and fetal biophysical profile score 8/10. Peripheral complete blood counts (CBC) revealed marked anemia, mild leukocytosis and severe thrombocytopenia. Her CBC evolution can be seen in Table 1. The blood film demonstrated significant (11 to 13%) schistocytes, consistent with MAHA. Further laboratory investigations were consistent with hemolysis: lactate dehydrogenase of 1.089 U/L (normal range 125 to 220 U/L), absolute reticulocytes count of 219.000/uL (normal range between 16.400 - 77.600/uL), indirect bilirubin of 1.1mg/dL (normal range until 0.7 mg/dL), and negative direct antiglobulin testing (DAT). Coagulation tests, renal function and liver enzymes were between normal values.
Complete blood count evolution during hospitalization.
WBC: White blood count; Hb: hemoglobin; HCT: hematocrit; MCV: mean corpuscular volume; PLT: platelets; LDH: Lactate dehydrogenase.
The cranial scan did not show any abnormality and the fetal evaluation was normal. The patient's findings were consistent with TMA and possibly TTP as her PLASMIC Score was 6 points (high risk), showing a 72% chance of having severe ADAMTS13 deficiency. ADAMTS 13 dosing is not available in the public system of Brazil, even with stored plasma. The patient was promptly submitted to therapeutic plasma exchange (PEX), associated with high dose methylprednisolone for three days, and then prednisone started at 1 mg/kg per day. During the further investigation, she also tested positive for HIV. The CD4 lymphocyte count was 184/uL (540 - 1660 cells/uL) and treatment with Tenofovir 400 mg, Lamivudine 300 mg and Dolutegravir 50 mg once daily was started on day four of the PEX treatment.
During the hospitalization period, the patient was submitted to daily PEX for five days, until the normalizing of LDH and platelets for two consecutive days. Afterwards, she continued PEX every other day for two sessions more and stopped when achieving a complete response. She was submitted to a total of 7 PEX sessions. The LDH and platelets at the end of the PEX were 191U/L and 179.000/uL, respectively. Patients and fetus were daily monitored by the obstetric team through maternal physical examination, fetal heartbeats and movements during all shifts, showing adequate development throughout the hospitalization. She was discharged after thirteen days with oral prednisone with total recovery. The glucocorticoid tapering was made over two weeks with no signs of clinical or laboratorial recurrence. She underwent a cesarean delivery due to induction failure with 39 weeks and 6 days, male newborn, 3710 grams and APGAR score were 8 and 9 points (1minute/5minutes). She had normal complete blood counts during labor and later consultations.
DiscussionTo the best of our knowledge, this is the first report in which HIV infection was diagnosed during pregnancy in concomitance with a TMA. It is challenging to determine how much each condition (HIV and pregnancy) has contributed to its development. Ranzini et al5 had already described an HIV infection embroiling TTP during pregnancy, but the patient was already known to be HIV positive. Pregnancy has been recognized as a high-risk period for developing TMA. Furthermore, it may trigger a TTP event in patients with cTTP or in those with acquired TPP and persistent ADAMTS13 deficiency. Others cohorts addressed a higher incidence on the third trimester, although a more recent cohort from the United Kingdom showed that the disease is more frequent during the second trimester.6,7 It is remarkable that this cohort used ADAMTS13 dosing to confirm the diagnosis, which was not made on the previous cohorts.
The findings of MAHA in the gestational period have to initiate a process of suitable differential diagnosis. The additional investigation made for the reported patient was capable of ruling out disseminated intravascular coagulation (DIC), uremic hemolytic syndrome (UHS) and HELLP syndrome. Moreover, clinical manifestations of hypertension, acute liver damage, autoimmune diseases and invasive diarrhea were lacking. Transient focal neurologic findings such as those presented by our patient (paresthesia in upper limb and left facial side) can occur in about 30% of patients with TTP.3
A central discussion of TTP related to pregnancy is the fact that this could be a late presentation of a cTTP. In the UK cohort,7 65% of patients with TTP in pregnancy were shown to have a congenital form. In case a congenital TTP is diagnosed, preemptive treatment during further gestations is recommended. There are some descriptions of the use of a purity factor VIII concentrate, which contains a high quantity of ADAMTS13.7 On the other hand, in case the diagnosis is aTTP, monitoring ADAMTS13 levels before and during pregnancy is an important approach. In relationship to the present case, it is significant to describe that the patient already had a normal pregnancy, without manifestations of MAHA. This clinical behavior diminishes the chance of a congenital form of the disease. In Brazilian public health system reality, ADAMTS13 dosing is not available to confirm diagnosis. Although this testing is fundamental to confirm diagnosis, costs and the involved technology are limited in the public health system in our country. Thus, it is important to rely it in clinical scoring systems and the urgency of treatment in our clinical practice.
HIV is likewise related to an acquired ADAMTS13 deficiency. An African cohort estimated that the incidence of TTP in HIV-infected individuals to be 15-40 times higher than non-infected individuals.8 There is a possible mechanism related to the inflammatory process of HIV and the development of TTP. It seems that cytokines stimulate the release of extreme amounts of Von Willebrand factor.9 Extremely high levels of VWF were found in plasma of HIV cases and in TTP plasma after PEX. Those inflammatory cytokines also downregulate the release of ADAMTS13. In HIV related TTP, the initiation of antiretroviral therapy should be done in parallel with PEX.
HIV screening is a routine in prenatal care. However, the patient in question wasn't performing regular prenatal care, not being tested for conventional tests. The absence or incompleteness of prenatal care is prevalent in Brazil, especially in the needy population, like the patient in question. After this event, besides having started anti-retroviral therapy (ART), she remained adherent to it. Besides PEX as a treatment for the acute phase of the disease, ART is the mainstay for preventing relapse.
Although ADAMTS13 that is the standard test for the PTT diagnosis, is not a reality in many developing countries on latin america. The present case highlight the possibility of making a fast and likely diagnosis based on clinical features, laboratories findings, epidemiology and specific scores such as PLASMIC, as this score was validated for rapid assessment of adults with TMA, as showed by Bendapudi et al.,10 where they assessed using internal and external validation cohorts and compared to clinical assessment alone. Being a singular disease, the clinical suspicion should be high to establish diagnosis. Even in the pregnancy scenario and the possibility of having other disturbances to diagnose (such as HIV in the actual case), the goal should be the prompt start of PEX.