Cancer patients are at a high risk of severe illness from COVID-19 and have been observed to have a mortality rate of over 25 %.1,2 This has led several groups to recommend delaying or deferring curative chemotherapy to minimize the risk of mortality due to severe COVID-19. However, delays in treating patients with hematologic malignancies, especially those with acute leukemia, are associated with poor outcomes.1
Following chemotherapy for acute myeloid leukemia (AML), complete remission (CR) is defined as having a blood neutrophil count of ≥1000/µL, platelets of ≥100,000/µL, no circulating blast cells, no presence of Auer rods, bone marrow blasts accounting for less than 5 %, absence of extramedullary disease (CNS and soft tissues), and no need for blood product transfusions.3
Spontaneous remission (SR) without chemotherapy, whether complete or partial, is an exceedingly rare occurrence and is typically of short duration.4 The first documented case of SR was reported after Eisenlohr's typhoid infection in 1878.5 Various events, such as infection and blood transfusion, have been suggested to play a significant role in SR. Nevertheless, the exact mechanism behind this phenomenon remains to be determined.6 One hypothesis posits that immune activation triggered by these events has anti-leukemic effects. Recent advancements in early diagnosis and treatment have made SR a rare phenomenon. 7
We present a case of M4 acute myelomonocytic leukemia (AMML) without cytogenetic abnormalities, in which spontaneous remission (SR) occurred after a severe coronavirus infection, along with multiple blood transfusions, without the use of chemotherapy.
Case reportDuring June 2020, a 25-year-old woman was admitted to a medical center in Cali, Colombia due to acute symptoms of abdominal pain, non-dysenteric diarrhea, fever, and seizures. She reported experiencing hyporexia and unquantified weight loss for the past six months. Additionally, she had a history of a convulsive syndrome attributed to a poroencephalic cyst. Upon admission, her vital signs were stable, and the only discomfort observed was upon palpation in the left flank, with no masses or megalia and without signs of peritoneal irritation. Her neurological examination did not reveal any relevant findings.
Upon admission, cerebrospinal fluid analysis showed no abnormalities, elevated C-reactive protein (CRP), moderate normocytic normochromic anemia, neutropenia, thrombocytopenia, and normal coagulation, liver, kidney, hydroelectrolytic, metabolic, endocrine, and tumor lysis profiles. Initial blood cultures yielded negative results, and serologies for HBV, HCV, and HIV were also negative. A right frontal poroencephalic cyst was confirmed, while chest X-ray, abdominal ultrasound, and echocardiogram results were normal.
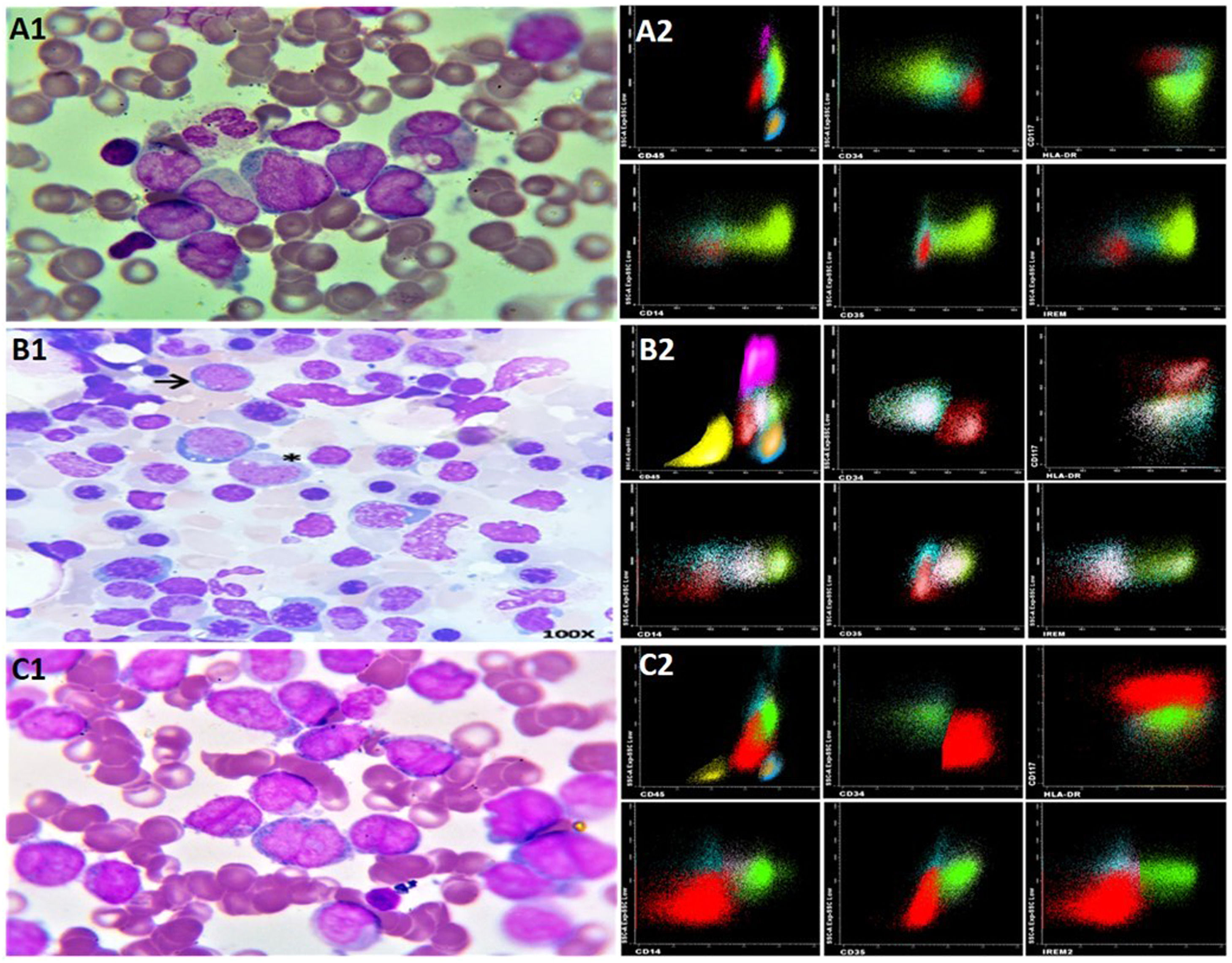
On the second day of hospitalization, she developed a fever with suspected sepsis of gastrointestinal origin. A peripheral blood smear showed monocytoblasts with the presence of Auer rods and monocytosis. Morphologically, M4 acute myelomonocytic leukemia (AML) was suspected according to the French-American-British classification (Figure 2A1).
By the fourth day, peripheral blood flow cytometry provided an immunophenotypic diagnosis of acute myelomonocytic leukemia (AML) (Figure 2A2). Cytogenetic analysis revealed a 46 XX karyotype with no chromosomal abnormalities. A wild-type FLT3 ITD – TKD was reported, leading to its cytogenetic classification as a favorable risk AML.
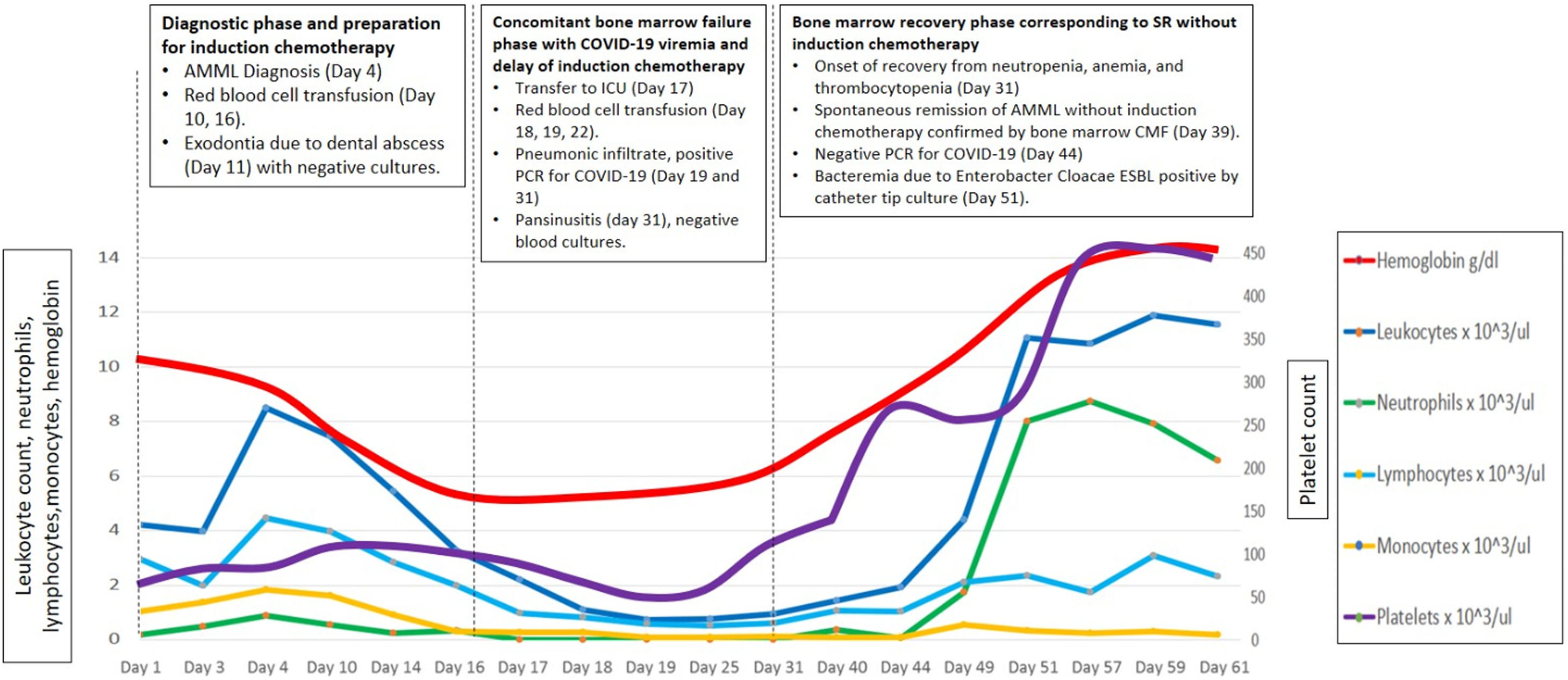
On day 11, in preparation for induction chemotherapy, wisdom tooth removal was complicated by an abscess that required drainage and debridement of the granulomatous tissue. Tissue cultures and blood cultures returned negative results. However, by day 17, her bone marrow failure worsened, leading to febrile neutropenia (Figure 1). To address this, she was admitted to the Intensive Care Unit (ICU), and broad-spectrum antibiotics, antifungals, and multiple red blood cell transfusions (a total of 10 units) were initiated due to persistent anemia.
On day 19, her marrow failure deepened, and she simultaneously developed respiratory symptoms. A high-resolution computerized axial tomography (HRCT) scan revealed "ground glass" opacities with a tendency toward consolidation in both lung fields. Additionally, inflammatory markers such as lactate dehydrogenase, C-reactive protein, ferritin, and d-dimer were persistently and severely elevated. A PCR test for COVID-19 returned positive on day 19, prompting the initiation of treatment with low-dose dexamethasone (8 mg daily intravenously for ten days) and colchicine (1 mg orally every 12 h) due to pulmonary inflammation and the risk of fibrosis.
By day 39, her hemogram had returned to normal (see Figure 1). A follow-up flow cytometry analysis of the bone marrow revealed less than 1 % of myeloid precursors and less than 5 % of monocytic line presence, with no immunophenotype for LAMA detected. Considering the hematological, morphological, and immunophenotypic response, it was determined that no further treatment was required (see Figure 2, panels B1 and B2). On day 44, the PCR test for COVID-19 came back negative, and she was discharged after 61 days.
Correlation between morphology of AMML M4 and immunophenotype. (A1) BM Dx: AMML M4 (A2) FC Dx: immature myeloid CD13+/CD34+/CD117+/HLA-DR+, CD45+/-CD64+/-CD16-/CD11b-/CD10-/CD35-/CD14- /IREM- (red) and HLA-DR+/CD13+ /CD64+ monocytes with 2 populations CD35-/CD14-/IREM- monoblasts (Aquamarine) and CD35+/CD14+/IREM+ monocytes (Fluorescent Green) (B1) BM remission: few blasts → monocytes * (B2) FC BM: no AMML (C1) BM relapse: AMML M4 (C2) FC BM relapse: AMML. Dx: diagnosis, BM: bone marrow, FC: flow cytometry, AMML: acute myelomonocytic leukemia.
In December 2020, after 5 months of follow-up, she began to experience B symptoms and developed generalized lymphadenopathies. A lymph node biopsy from her neck was positive for CD34, CD117, CD68, MPO, CD4, and Focal TdT but negative for CD20, CD3, CD79a, with a Ki67 rate of 90 %. A bone marrow flow cytometry with an immunophenotype similar to the initial diagnosis confirmed AMML relapse (see Figure 2, panels C1-C2). Treatment with cytarabine and daunorubicin (7 + 3) achieved minimal residual disease. Unfortunately, consolidation with high doses of cytarabine was not possible due to drug shortages in the country. Almost one year after achieving the first complete remission (CR1), the bone marrow transplant group decided not to proceed with a transplant, opting for outpatient follow-up.
In March 2022, 21 months later of the first episode, she was hospitalized again due to a relapse of her AMML. High-dose cytarabine (HIDAC) treatment was initiated. Subsequently, she underwent allogeneic bone marrow transplantation from an HLA-matched sibling donor using reduced-intensity conditioning with fludarabine and busulfan. The patient is currently under follow-up and is disease-free.
DiscussionSpontaneous remission (SR) in AML is rare and is typically associated with bacterial infections.7 In this case, the coronavirus infection occurred simultaneously with a period of severe neutropenia. During this time, there was a notable shift in the phenotype of the leukemic blasts, characterized by a loss of immature monocytic markers. However, this period of remission was short-lived, lasting less than 6 months. Subsequently, the monoblastic immature pathological immunophenotype reemerged after a viremic state caused by COVID-19.
The downregulation of peripheral monoblasts during SR may be attributed to a "silencing" effect that occurs during viral infections in leukemic blast clones.8 These clones can manage to survive until relapse, aligning with hypotheses related to immune-mediated phenomena in AML. These hypotheses encompass processes such as the blockade of the IFN pathway, the JAK-STAT pathway in leukemic cells,8 tissue recruitment by inflammatory macrophages,9 elevated levels of IL-2 and GM-CSF,10 and the graft-versus-leukemia/lymphoma (GVL) effect facilitated by the transfusion of non-irradiated blood products,11 among others.
Taking the above into account, we will now discuss the following six hypotheses associated with the SR in this case:
Hypothesis 1 The coronavirus controls the adaptive viral response by inhibiting the IFN pathway through the blocking of TLR and RLR receptors and the downregulation of nuclear transcription factors. This is achieved by hijacking JAK-STAT linker proteins, which are an important signaling pathway for the growth of AMML clones. Interferon I and III (IFN I and III) bind to IFNAR1 and IFNLR receptors, respectively, on inflammatory cells by recognizing viral RNA. They activate interferon-stimulating genes (ISGs) through Toll-like receptors (TLR) and RLR, promoting signals in the JAK/STAT and NF-κB pathways, which are the most studied pathways in leukemia.12 Coronavirus subgenomic RNAs (structural and nonstructural) sequester JAK-STAT connecting proteins, inhibiting phosphorylation and translocation of transcription factors nuclear IFR1 and IRF3, thus deactivating ISGs, blocking IFN signaling, production, and response.8
Hypothesis 2 In AMML and severe COVID-19, inflammatory macrophages in alveolar tissues induce chemotaxis, sequestration, and peripheral depression of leukemic monocytes, NK cells, and lymphocytes, changing them to an inflammatory CXCR3+ phenotype. In patients with severe COVID-19, alveolar tissue macrophages exhibit high chemotaxic activity due to the expression of CXCR3 and its ligands CXCL9/CXCL10/CXCL11. The virus activates inflammatory macrophages, which recruit monocytes, T cells, and NK cells, transforming them into an inflammatory CXCR3+ phenotype.9
Hypothesis 3 Elevated IL-2 levels in severe COVID-19 may downregulate AMML cells through an antitumor effect upon the activation of cytotoxic T and NK cells. In severe COVID-19 infections, IL-2 levels are elevated, and the interaction between IL-2 and its receptor (IL-2R) on peripheral blood mononuclear cells (PBMCs) activates CD4+ and CD8+ T lymphocytes, as well as NK cells. However, in patients with critical COVID-19, there is a decrease in the expression of IL-2R and JAK1-STAT5 in PBMCs, which can lead to increased immunosuppression.13 Recombinant IL-2 has been used as a biological response modifier to activate CD8+ T lymphocytes and NK cells against AML blast cells in consolidation, relapse, and second relapse therapy.14
Hypothesis 4 Cross-immunity induced by the coronavirus may control AMML clones by stimulating G-CSF production. Patients with severe COVID-19 exhibit elevated levels of monocytes and CD4+ Th1 cells expressing IL-2, IL-6, granulocyte colony-stimulating factor (G-CSF), and IFN-γ.13 G-CSF can suppress leukemic cell clones by inducing apoptosis.14 Cases of SR in AML without chemotherapy after applying recombinant granulocyte colony-stimulating factor (rhG-CSF) during associated infections have been published, demonstrating improvement of the AML infection and achieving complete remission.10
Hypothesis 5 Transfusion of non-irradiated blood components may contribute to RS AMML, potentially through a graft-versus-leukemia (GVL) effect. Transfusion of non-irradiated blood products can potentially induce antileukemic effects, such as the graft-versus-leukemia (GVL) effect, by transfusing allogeneic lymphocytes that produce cytotoxic antibodies and cytokines capables of inducing RS in AML.11
Hypothesis 6 Adjuvant treatment for COVID-19 with colchicine, due to its anti-microtubule effect, may slow cell division in AMML cells. Colchicine exerts an antiproliferative and anticancer effect by inhibiting mitosis, halting the formation of microtubules, and inducing cell death through oxidative stress and DNA damage.15 While corticosteroids are anti-inflammatory agents that significantly reduce complications in severe cases of COVID-19 16, they do not have a defined therapeutic role in AML.
Spontaneous remission (SR) in AML is a rare occurrence, with a median reported remission period of 8.2 ± 9.8 months. Several potential causes have been identified, including infections, elevated levels of IL-2, granulocyte colony-stimulating factor (G-CSF) produced by inflammatory cells, transfusion of blood products, and drugs.6 In this context, the case under consideration experienced a disease-free period exceeding 5 months, which is somewhat longer than the median average SR duration of 5 months reported in the literature.
We propose the exploration of innovative treatment avenues. These may encompass investigating new generations of CAR T cell therapy using coronavirus vectors, harnessing the potential of allogeneic lymphocytes to enhance the graft-versus-leukemia (GVL) effect and the production of cross-reacting cytotoxic antibodies in donor serum for hemotherapy. Additionally, the utilization of recombinant soluble factors, such as TNF, IL-2, IFN, and G-CSF, with a focus on toxicity control, as well as the exploration of immunotherapy with tumor-specific T and NK cells, represents a diverse array of theoretical possibilities inspired by the unique characteristics of this case.
The authors of this publication extend their heartfelt gratitude to the hospitalization care service group and the intensive care unit at the Belarcazar Colsanitas S.A. clinic, part of Grupo Keralty, located in Cali, Colombia.