Immune thrombotic thrombocytopenic purpura (iTTP) is characterized by acute systemic microvascular thrombosis and is associated with a high morbidity and mortality, especially in delayed diagnosis (later than 6–7 days from symptoms). iTTP data in Brazil is scarce, so we aimed to characterize the clinical presentation and identify predictors of death risk in patients with this disease in Brazil.
MethodsIn this single-center retrospective study the patients who underwent therapeutic plasma exchange (TPE) for presumptive or confirmed iTTP were evaluated regarding the epidemiological, clinical, laboratorial characteristics and management.
ResultsA total of 50 patients (90 % female), with median age (IQR) of 34.1 (27–47) years, were enrolled, of which 12 (24 %) died. The most frequent symptoms were neurological (96 %), bleeding (76 %), gastrointestinal (52 %), fever (38 %), and cardiovascular (22 %). Neurological focal deficit and cardiovascular symptoms were more frequently observed in the non-survivor group (P = 0.0019 and P = 0.007, respectively). The mean ± SD number of days from beginning of symptoms to first TPE was 12.22 ± 7.91. We identified an association regarding mortality rate with a score MITS ≥ 2 points (P = 0.04), a higher indirect bilirubin (P = 0.0006), a higher number of transfused red blood cell units (P = 0.025), and platelet transfusion (P = 0.027).
ConclusionDelayed diagnosis appears to be associated with a higher frequency of neurological symptoms and mortality. Intensity of hemolysis and signs of organ ischemia, such as cardiovascular symptoms and focal neurological deficit, are indicators of death risk.
Thrombotic thrombocytopenic purpura (TTP) is a rare and potentially fatal disease characterized by acute microvascular systemic thrombosis. It is caused by severe deficiency of ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type 1 motifs, member 13), which leads to the accumulation of ultra-large von Willebrand factor resulting in its increased adhesion to platelets and the formation of platelet-rich microthrombi in microvasculature.1,2 The consequences are thrombocytopenia, microangiopathic hemolytic anemia and organ ischemia.1,2 Visceral ischemia presents as multiorgan damage, such as neurologic, cardiac, mesenteric and renal.3,4 In most cases, the cause of TTP is the formation of autoantibodies against ADAMTS13 (iTTP). More rarely, the deficiency is due to genetic mutations resulting in a lower synthesis of the enzyme.5-7
The diagnosis of TTP is made with an ADAMTS13 activity lower than 10 %. Nevertheless, due to the therapeutic urgency, treatment must be initiated as soon as possible, usually before ADAMTS13 dosing, and based on presumptive diagnosis by the clinical and laboratory features. The application of diagnostic scores, such as Plasmic and French, can be decisive to indicate therapeutic plasma exchange (TPE), before the result of ADAMTS13 quantification, when this test is available, and especially when it is not.2,8,9
The mortality rate up to a few decades ago was, approximately, 90 %,10 however, it dropped to 10 a 20 % with the adoption of TPE as standard treatment.11 Immunosuppression associated with the TPE is recommended by most authors. Glucocorticoids and/or rituximab are the most used agents for this purpose.12,13 Another therapeutic option, proposed more recently, is the upfront administration of caplacizumab, a fragment humanized immunoglobulin which inhibits the binding between platelets and von Willebrand factor.14
There are factors that have been associated with higher mortality, such as age, severe cerebral involvement, high lactate dehydrogenase (LDH) level, arterial thrombosis, intracranial hemorrhage, renal failure, platelet transfusion and cardiovascular complications.15–19 Two predictive models for death risk were developed according to the aforementioned conditions: French TMA Reference Center Score and Mortality in TTP score (MITS).15,18 iTTP was also associated with a high morbidity, especially in delayed diagnosis (beyond 6–7 days), which has been associated with increased neurological complications.20 iTTP data in Brazil are scarce, with just a few studies conducted so far. To the best of our knowledge, this is the largest study carried out in this country and it is important for providing data from our population, which could be used in the future as a comparing basis for new therapies, such as caplacizumab.
In this study, we aimed to characterize the clinical and laboratorial presentation, management, and predictors of death risk in patients with immune TTP admitted to a single center in Brazil.
Materials and methodsStudy design and participantsThis is a retrospective observational study conducted at Hospital das Clínicas de Ribeirão Preto, affiliated to the medical school of Ribeirão Preto, University of São Paulo (USP). This is a tertiary public hospital with 815 beds. This study included patients with iTTP, presumptive or confirmed, treated with TPE between January 2000 and July 2021. We included data of patients with de novo or relapsed iTTP. The identification of patients was performed from TPE medical charts from the apheresis service of Regional Blood Center of Ribeirão Preto, University of São Paulo. Exclusion criterion was: diagnosis attributed to other conditions. The variables were compared between survivor and non-survivor groups.
Recorded variables and definitionsThe diagnosis of iTTP was defined as the presence of microangiopathic hemolytic anemia associated with thrombocytopenia and a clinical presentation consistent with TTP. A minority of patients had ADAMTS13 activity and inhibitor investigation documented. We considered as having iTTP the patients with compatible clinical presentation, including the application of Plasmic and French scores, or confirmed by severe (< 10 %) or low levels of ADAMTS13 (10–20 %) with positive inhibitor to this enzyme.21
The participants were evaluated for age, sex, period of admission, time to admission, cardiovascular risk factors (smoking, arterial hypertension, obesity, diabetes and ischemic stroke), human immunodeficiency virus (HIV) infection and current pregnancy. Plasmic and French scores were evaluated in patients with de novo TTP.8,9
Clinical presentation was classified into the following categories: fever at admission; bleeding (mucocutaneous, gastrointestinal and central nervous system); neurological symptoms (headache, focal deficit, altered level of consciousness and seizure); creatinine > 2 mg/dL at admission; acute ischemic stroke; venous thromboembolism; cardiovascular symptoms (cardiogenic pulmonary edema, chest pain and bradycardia); gastrointestinal symptoms (nausea and vomiting, abdominal pain and diarrhoea).
Clinical response was defined as a sustained platelet count ≥ 150 × 109/L and LDH < 1.5 times the upper limit of normal, with no clinical evidence of new or progressive ischemic organ injury.6,22 Clinical exacerbation was defined as a decrease of platelet count to < 150 × 109/L (with other causes of thrombocytopenia excluded) within 30 days from interrupting TPE.7,28 Relapse was a reduction in platelet count to < 150 × 109/L (with exclusion of other causes of thrombocytopenia) after a clinical remission (sustained clinical response with no TPE in the past 30 days) was achieved.6,22
Laboratory proceduresRoutine blood tests were: complete blood count, reticulocyte count, coagulation profile, direct bilirubin and indirect bilirubin, lactate dehydrogenase (LDH), blood urea nitrogen (BUN), creatinine, alanine aminotransferase (ALT) and aspartate aminotransferase (AST). The ADAMTS13 activity and ADAMTS13 inhibitor investigation was performed in 17 patients. The laboratorial values used for the analyses were obtained on admission to the hospital.
Treatment scheduleTPE was initiated as soon as the diagnosis was suspected, and performed daily until clinical response was achieved. Before 2014, patients were occasionally treated with corticosteroids according to the clinical condition, such as severe clinical course, refractoriness, clinical exacerbation and relapse. After 2014, all patients, besides TPE, received corticosteroids as first-line therapy. Rituximab was used in cases of refractoriness or relapsed disease only after 2012, due to restricted availability of this agent in the public scenario in our country. None of the participants received caplacizumab.
Application of severity scoresTwo severity scores were applied: the French TMA Reference Center Score15 and Mortality in TTP score (MITS).18 The scores were only applied to the 44 patients with de novo TTP, according to the studies that proposed them. The French TMA Reference Center Score includes the items: cerebral involvement (1 point), LDH level 10 times the referral limit or over (1 point), age between 41 and 60 years (1 point), and age over 60 years (2 points).15 MITS has the following scoring scheme: platelet transfusion (1 point), acute myocardial infarction (1 point), ischemic stroke (1 point), renal failure (acute renal failure, chronic, or hemodialysis) (1 point), age (≥ 60 years of age) (2 points), CNS bleed (3 points), and arterial thrombosis (3 points).18
Data collectionEpidemiological, clinical, laboratory, treatment, and outcome data were extracted from the medical charts using a standardized data collection form.
Statistical analysisContinuous variables were expressed as means and standard deviation (SD) or medians and interquartile range (IQR), according to distribution characteristics (the Kolmogorov–Smirnov test was employed to define distribution characteristics). Categorical variables were summarized as the counts and percentages in each category. To compare quantitative variables between the two groups a Student's t-test was used if the variables had Gaussian distribution, and a Mann–Whitney U test when they did not. Statistical comparison between groups for categorical variables were performed by employing a chi-square or Fisher exact test. A log-binomial regression model was used to estimate the relative risk through the univariate model. Multivariate analysis was not possible due to the small sample size. Survival analysis was performed using the Kaplan-Meier method and the difference between groups was assessed using the log-rank test. The results were considered to be statistically different when the P-value was below 0.05. Statistical analyses were performed using statistical GraphPAD Prism Software, version 9.1 and SAS Analytics software, version 9.4.
Ethical considerationThis study was approved by the institutional review board (Comitê de Ética em Pesquisa do Hospital das Clínicas de Ribeirão Preto) (40,192,320.1.0000.5440), and was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. As this is a retrospective study, the ethical review board agreed to waiver the signed consent by the patients.
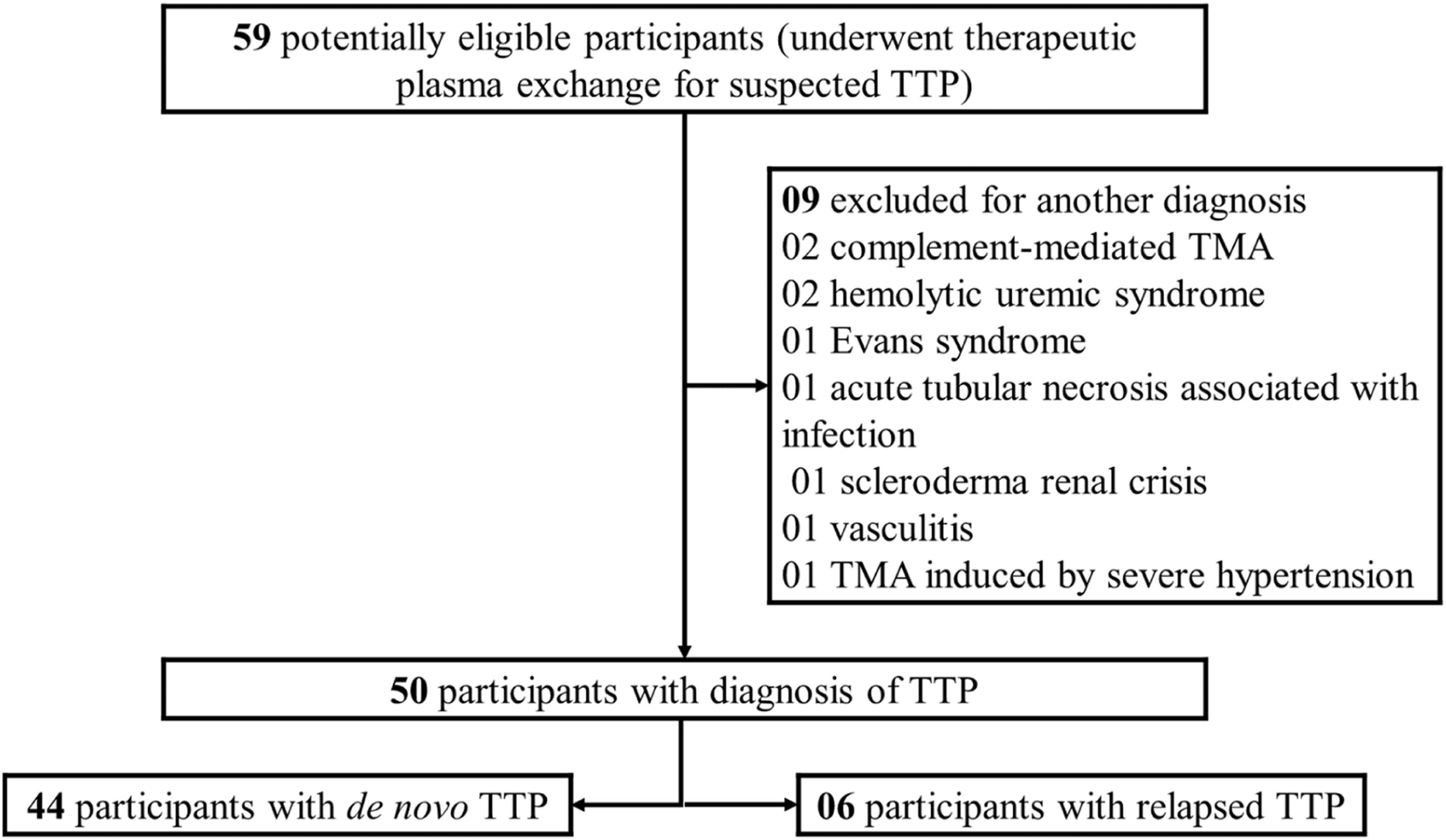
ResultsA total of 59 patients with thrombotic microangiopathy, whose main diagnostic hypothesis was iTTP, underwent TPE and were potentially eligible for the study. Nine patients were excluded because they received another diagnosis during hospitalization (Figure 1). The remaining 50 patients (90 % females) were considered as having iTTP, of which 44 patients (88 %) had de novo iTTP and 6 patients (12 %) had relapsed iTTP. Plasmic and French scores were applied to 44 patients with de novo iTTP. Five (11.4 %) of them had a Plasmic score of 5 points and 39 (88.6 %) scored from 6 to 7 points. Regarding the French score, 3 (6.8 %), 31 (70.5 %) and 10 (22.7 %) patients presented, respectively, 1, 2, and 3 points. The mean ± SD number of days from beginning of symptoms to first TPE was 12.22 ± 7.91. None of the participants was pregnant at the presentation.
ADAMTS 13 activity and inhibitor investigation tests were performed in 17 patients, of which 16 (94 %) had ADAMTS13 activity < 10 % and 15 (88.2 %) had detectable ADAMTS13 inhibitors. One patient had low ADAMTS 13 activity (18 %) with a positive inhibitor.
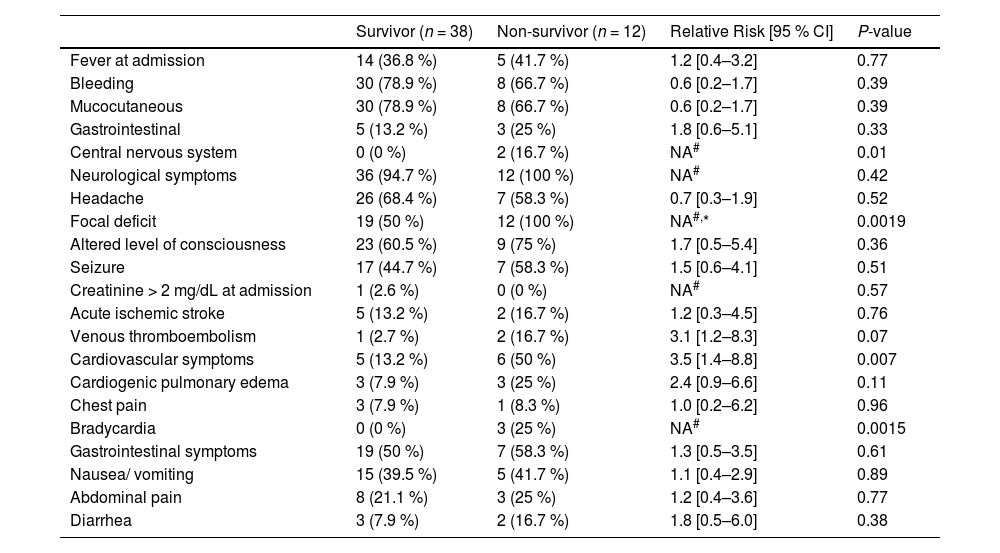
A total of 12 patients (24 %) died. Mean ± SD days for death was 12.5 ± 9.5 days after admission. Baseline characteristics of patients are depicted in Table 1 and clinical presentation in Table 2. We did not observe a difference in mortality rate between the group of patients admitted from 2000 to 2010 and the group of the patients admitted from 2011 to 2021 (26.3% vs 22.6 %; P = 0.76). Central nervous system bleeding, neurological focal deficit, cardiovascular symptoms and bradycardia were more frequently observed in the non-survivor group.
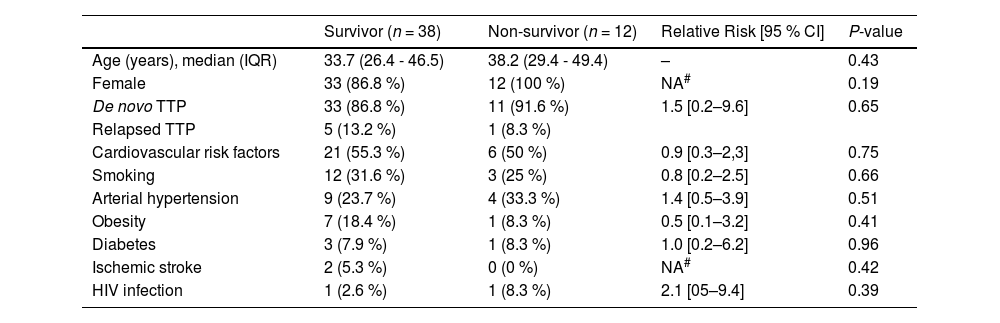
Baseline characteristics according to outcome (survivor and non-survivor).
| Survivor (n = 38) | Non-survivor (n = 12) | Relative Risk [95 % CI] | P-value | |
|---|---|---|---|---|
| Age (years), median (IQR) | 33.7 (26.4 - 46.5) | 38.2 (29.4 - 49.4) | – | 0.43 |
| Female | 33 (86.8 %) | 12 (100 %) | NA# | 0.19 |
| De novo TTP | 33 (86.8 %) | 11 (91.6 %) | 1.5 [0.2–9.6] | 0.65 |
| Relapsed TTP | 5 (13.2 %) | 1 (8.3 %) | ||
| Cardiovascular risk factors | 21 (55.3 %) | 6 (50 %) | 0.9 [0.3–2,3] | 0.75 |
| Smoking | 12 (31.6 %) | 3 (25 %) | 0.8 [0.2–2.5] | 0.66 |
| Arterial hypertension | 9 (23.7 %) | 4 (33.3 %) | 1.4 [0.5–3.9] | 0.51 |
| Obesity | 7 (18.4 %) | 1 (8.3 %) | 0.5 [0.1–3.2] | 0.41 |
| Diabetes | 3 (7.9 %) | 1 (8.3 %) | 1.0 [0.2–6.2] | 0.96 |
| Ischemic stroke | 2 (5.3 %) | 0 (0 %) | NA# | 0.42 |
| HIV infection | 1 (2.6 %) | 1 (8.3 %) | 2.1 [05–9.4] | 0.39 |
CI, confidence interval; HIV, human immunodeficiency virus. IQR, interquartile range; NA, not applicable; TTP, thrombotic thrombocytopenic purpura.
Clinical presentation of the survivor and non-survivor groups.
| Survivor (n = 38) | Non-survivor (n = 12) | Relative Risk [95 % CI] | P-value | |
|---|---|---|---|---|
| Fever at admission | 14 (36.8 %) | 5 (41.7 %) | 1.2 [0.4–3.2] | 0.77 |
| Bleeding | 30 (78.9 %) | 8 (66.7 %) | 0.6 [0.2–1.7] | 0.39 |
| Mucocutaneous | 30 (78.9 %) | 8 (66.7 %) | 0.6 [0.2–1.7] | 0.39 |
| Gastrointestinal | 5 (13.2 %) | 3 (25 %) | 1.8 [0.6–5.1] | 0.33 |
| Central nervous system | 0 (0 %) | 2 (16.7 %) | NA# | 0.01 |
| Neurological symptoms | 36 (94.7 %) | 12 (100 %) | NA# | 0.42 |
| Headache | 26 (68.4 %) | 7 (58.3 %) | 0.7 [0.3–1.9] | 0.52 |
| Focal deficit | 19 (50 %) | 12 (100 %) | NA#,* | 0.0019 |
| Altered level of consciousness | 23 (60.5 %) | 9 (75 %) | 1.7 [0.5–5.4] | 0.36 |
| Seizure | 17 (44.7 %) | 7 (58.3 %) | 1.5 [0.6–4.1] | 0.51 |
| Creatinine > 2 mg/dL at admission | 1 (2.6 %) | 0 (0 %) | NA# | 0.57 |
| Acute ischemic stroke | 5 (13.2 %) | 2 (16.7 %) | 1.2 [0.3–4.5] | 0.76 |
| Venous thromboembolism | 1 (2.7 %) | 2 (16.7 %) | 3.1 [1.2–8.3] | 0.07 |
| Cardiovascular symptoms | 5 (13.2 %) | 6 (50 %) | 3.5 [1.4–8.8] | 0.007 |
| Cardiogenic pulmonary edema | 3 (7.9 %) | 3 (25 %) | 2.4 [0.9–6.6] | 0.11 |
| Chest pain | 3 (7.9 %) | 1 (8.3 %) | 1.0 [0.2–6.2] | 0.96 |
| Bradycardia | 0 (0 %) | 3 (25 %) | NA# | 0.0015 |
| Gastrointestinal symptoms | 19 (50 %) | 7 (58.3 %) | 1.3 [0.5–3.5] | 0.61 |
| Nausea/ vomiting | 15 (39.5 %) | 5 (41.7 %) | 1.1 [0.4–2.9] | 0.89 |
| Abdominal pain | 8 (21.1 %) | 3 (25 %) | 1.2 [0.4–3.6] | 0.77 |
| Diarrhea | 3 (7.9 %) | 2 (16.7 %) | 1.8 [0.5–6.0] | 0.38 |
CI, confidence interval; NA, not applicable.
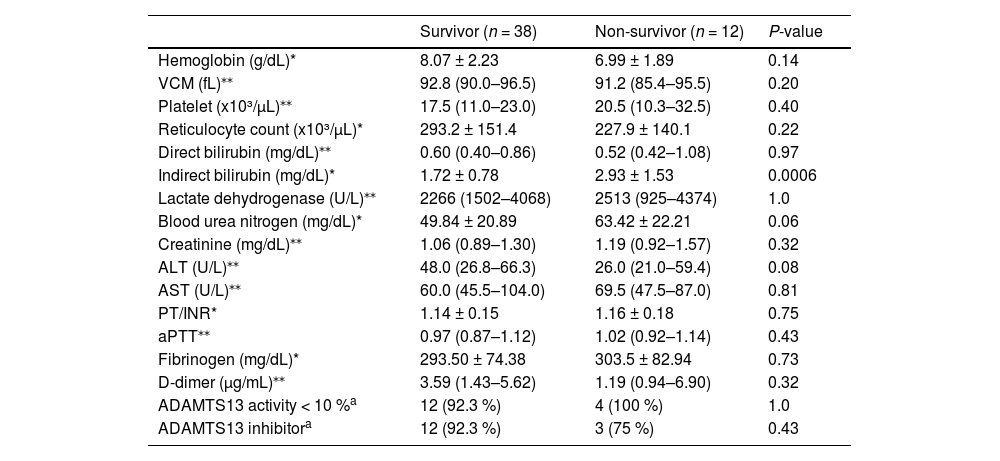
In relation to laboratory features at admission, the non-survivor group presented a higher indirect bilirubin. Other parameters were similar between the groups (Table 3).
Comparison laboratory features at admission between the two groups, survivor and non-survivor.
| Survivor (n = 38) | Non-survivor (n = 12) | P-value | |
|---|---|---|---|
| Hemoglobin (g/dL)* | 8.07 ± 2.23 | 6.99 ± 1.89 | 0.14 |
| VCM (fL)⁎⁎ | 92.8 (90.0–96.5) | 91.2 (85.4–95.5) | 0.20 |
| Platelet (x10³/µL)⁎⁎ | 17.5 (11.0–23.0) | 20.5 (10.3–32.5) | 0.40 |
| Reticulocyte count (x10³/µL)* | 293.2 ± 151.4 | 227.9 ± 140.1 | 0.22 |
| Direct bilirubin (mg/dL)⁎⁎ | 0.60 (0.40–0.86) | 0.52 (0.42–1.08) | 0.97 |
| Indirect bilirubin (mg/dL)* | 1.72 ± 0.78 | 2.93 ± 1.53 | 0.0006 |
| Lactate dehydrogenase (U/L)⁎⁎ | 2266 (1502–4068) | 2513 (925–4374) | 1.0 |
| Blood urea nitrogen (mg/dL)* | 49.84 ± 20.89 | 63.42 ± 22.21 | 0.06 |
| Creatinine (mg/dL)⁎⁎ | 1.06 (0.89–1.30) | 1.19 (0.92–1.57) | 0.32 |
| ALT (U/L)⁎⁎ | 48.0 (26.8–66.3) | 26.0 (21.0–59.4) | 0.08 |
| AST (U/L)⁎⁎ | 60.0 (45.5–104.0) | 69.5 (47.5–87.0) | 0.81 |
| PT/INR* | 1.14 ± 0.15 | 1.16 ± 0.18 | 0.75 |
| aPTT⁎⁎ | 0.97 (0.87–1.12) | 1.02 (0.92–1.14) | 0.43 |
| Fibrinogen (mg/dL)* | 293.50 ± 74.38 | 303.5 ± 82.94 | 0.73 |
| D-dimer (µg/mL)⁎⁎ | 3.59 (1.43–5.62) | 1.19 (0.94–6.90) | 0.32 |
| ADAMTS13 activity < 10 %a | 12 (92.3 %) | 4 (100 %) | 1.0 |
| ADAMTS13 inhibitora | 12 (92.3 %) | 3 (75 %) | 0.43 |
median (IQR); SD, standard deviation; VCM, mean corpuscular volume; IQR, interquartile range; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PT/INR, prothrombin time with international normalized ratio; aPTT, activated partial thromboplastin time; ADAMTS13, a disintegrin and metalloproteinase with thrombospondin type 1 motifs, member 13.
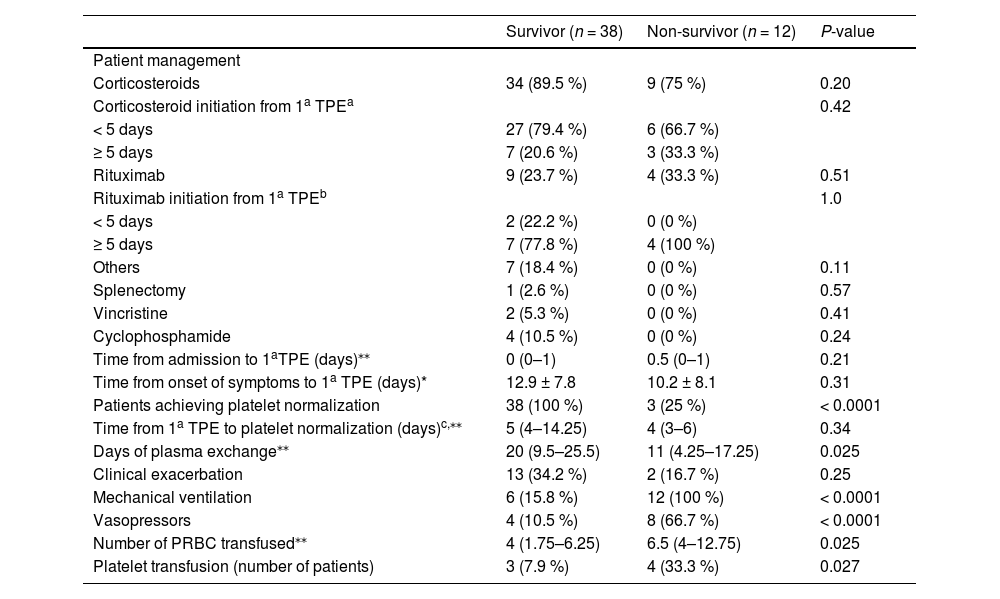
The patient management according to outcome is summed up in Table 4. All patients in both groups underwent TPE. There was no difference regarding time from admission to first TPE, use of corticosteroids, rituximab and other therapies between the two groups, survivor and non-survivor.
Patient management according to outcome.
| Survivor (n = 38) | Non-survivor (n = 12) | P-value | |
|---|---|---|---|
| Patient management | |||
| Corticosteroids | 34 (89.5 %) | 9 (75 %) | 0.20 |
| Corticosteroid initiation from 1a TPEa | 0.42 | ||
| < 5 days | 27 (79.4 %) | 6 (66.7 %) | |
| ≥ 5 days | 7 (20.6 %) | 3 (33.3 %) | |
| Rituximab | 9 (23.7 %) | 4 (33.3 %) | 0.51 |
| Rituximab initiation from 1a TPEb | 1.0 | ||
| < 5 days | 2 (22.2 %) | 0 (0 %) | |
| ≥ 5 days | 7 (77.8 %) | 4 (100 %) | |
| Others | 7 (18.4 %) | 0 (0 %) | 0.11 |
| Splenectomy | 1 (2.6 %) | 0 (0 %) | 0.57 |
| Vincristine | 2 (5.3 %) | 0 (0 %) | 0.41 |
| Cyclophosphamide | 4 (10.5 %) | 0 (0 %) | 0.24 |
| Time from admission to 1aTPE (days)⁎⁎ | 0 (0–1) | 0.5 (0–1) | 0.21 |
| Time from onset of symptoms to 1a TPE (days)* | 12.9 ± 7.8 | 10.2 ± 8.1 | 0.31 |
| Patients achieving platelet normalization | 38 (100 %) | 3 (25 %) | < 0.0001 |
| Time from 1a TPE to platelet normalization (days)c,⁎⁎ | 5 (4–14.25) | 4 (3–6) | 0.34 |
| Days of plasma exchange⁎⁎ | 20 (9.5–25.5) | 11 (4.25–17.25) | 0.025 |
| Clinical exacerbation | 13 (34.2 %) | 2 (16.7 %) | 0.25 |
| Mechanical ventilation | 6 (15.8 %) | 12 (100 %) | < 0.0001 |
| Vasopressors | 4 (10.5 %) | 8 (66.7 %) | < 0.0001 |
| Number of PRBC transfused⁎⁎ | 4 (1.75–6.25) | 6.5 (4–12.75) | 0.025 |
| Platelet transfusion (number of patients) | 3 (7.9 %) | 4 (33.3 %) | 0.027 |
median (IQR); IQR, interquartile range; TPE, therapeutic plasma exchange; PRBC, packed red blood cell; SD, standard deviation.
Time of corticosteroid initiation was evaluated in n = 43 patients (n = 34 in the survivor group and n = 9 in the non-survivor group).
All patients in the survivor group achieved platelet normalization, whereas only 25 % in the non-survivor group). The duration of TPE was longer in the survivor group. Mechanical ventilation and vasopressors were used more often in the non-survivor group. The non-survivor group received a higher number of packed red blood cells (PRBC). Besides, a higher percentage of patients in the non-survivor group received platelet transfusion (Table 4).
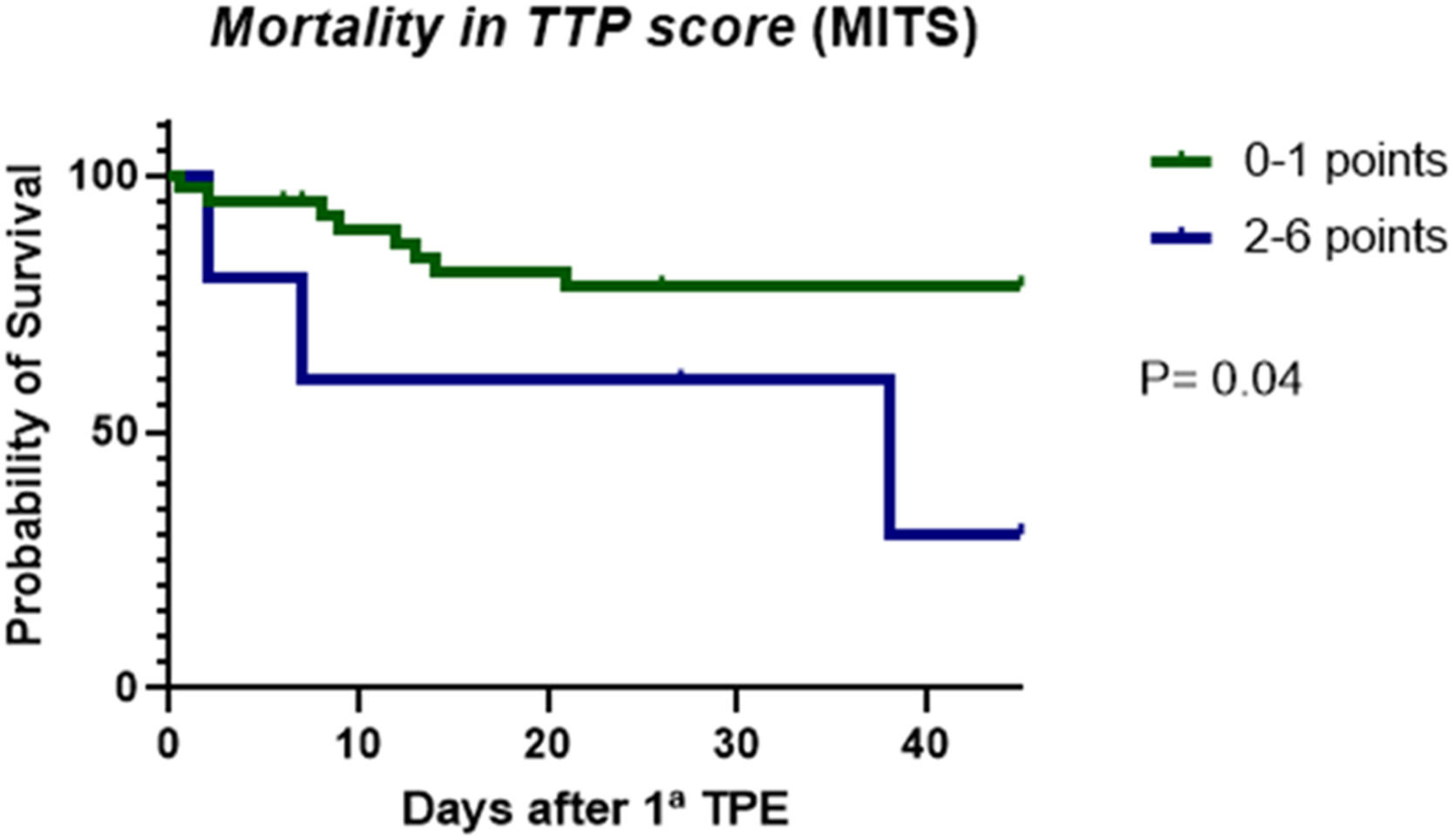
Regarding the application of MITS severity score system in the 44 patients with TTP de novo, we observed that the group of patients with score ≥ 2 points was associated with a lower probability of survival than the group with 0–1 points during hospitalization (Figure 2). However, applying the French TMA Reference Center score system did not show survival difference up to day 30 between the groups with scores 0–2 and 3–4 points (P = 0.26).
DiscussionIn this study, we show clinical and laboratorial presentation and management of immune TTP in 50 patients followed in a single center in Brazil. This study shows a death rate of 24 %, which is higher than that observed in British, Milan and Oklahoma registries in a similar period of time (8.5 %, 5 % and 13 %, respectively).4,23,24 A possible explanation for this finding could be the more extended time between the beginning of symptoms and diagnosis, which was approximately the double in our center (12 vs 6.5 days).20 Another study conducted in Brazil with patients with thrombotic microangiopathy, most with presumed TTP, also reported a prolonged time from the onset of symptoms to the first TPE in patients (mean of 19 days in the group of patients without neurological manifestations or mild symptoms and 17 days in the group with severe manifestations).25 We believe that the prolonged time between the onset of symptoms and the first TPE in our country may be related to several factors, such as: difficulty of diagnosing TTP, as it is a rare disease that requires recognition by the care team and need for a trained staff to visualize schistocytes on peripheral blood; difficulty in referral to a tertiary service (long distances to transport critically ill patients, limited availability of intensive care beds and specialized wards); few centers perform TPE in Brazil (for example, in our study, many patients were referred from other states).
Regarding the epidemiological characteristics, the median age of participants in our study was lower (34.1 years) and the prevalence of females (90 %) was higher than that described in other studies.3,4 In the French TTP Registry, the mean age was 43 and 68 % of participants were female.3 The UK TTP Registry reported a median age of 42 years and 75 % of patients were female.4
We found neurological symptoms in more than 90 % of the patients, a higher percentage than that reported by others, which was observed in, approximately, two-thirds of the patients.3,4,15 An explanation for this finding could be the longer period elapsed between the beginning of symptoms and the diagnosis (12 days) in our participants. Renaud et al. reported that a delayed diagnosis was associated with increased neurological events.20 Besides, we found that focal neurological deficit was associated with death risk. To the best of our knowledge, this finding was not previously reported as an independent factor of death risk. Benhamou Y et al. identified neurological involvement as a factor associated with poor prognosis and reported stupor and seizure, but not focal deficit, as independent factors associated with increased mortality.15
In our report, 22 % of patients had cardiovascular symptoms, which was associated with a higher death risk. A recent study also reported an association between in-hospital mortality and cardiovascular complications, which occurred in a quarter of patients.19 Cardiac involvement in TTP was rarely reported as the cause of death. Only after autopsy studies a high rate of cardiac involvement was revealed. Microvasculature is usually affected, with the presence of thrombi in arterioles, which results in myocardial hemorrhage and alterations in the conduction system.26 The most frequent clinical manifestations are chest pain, heart failure, electrocardiographic changes, troponin elevation, myocardial infarction, and sudden death.27
The frequency of fever and bleeding in our analysis was similar to other studies.3,23 Abdominal symptoms occurred in about half of the patients in our study, slightly higher compared to the French and British registries, which reported these symptoms in one third of patients.3,4
Laboratory features such as high LDH, D-dimer, total bilirubin and serum creatinine values on admission were previously reported to be associated with a higher death risk.15,28,29 In our analysis, LDH level was not significantly associated with mortality, however, higher indirect bilirubin, a hemolysis biomarker, was. This means that the intensity of hemolysis could, perhaps, be used as an indicator of death risk. D-dimers concentrations were not different between the groups, contrary to what others found, who suggested that elevated D-dimers was associated with a higher death risk.29 As expected, the absence of platelet normalization, the requirement of mechanical ventilation and the need of vasopressors administration were more frequently observed in the non-survivor group, which is compatible with the findings shown in other publications.28,30 Regarding blood transfusion, the non-survivor group received more packed red blood cells. Also, a higher number of patients in the non-survivor group received platelet transfusion, which was already demonstrated by Goel et al.20 More intense hemolysis and platelet consumption are obvious indicators of a more severe disease, which arguably requires more supportive measures, such as blood transfusion. For instance, more profound and prolonged thrombocytopenia may be associated with a higher risk of brain hemorrhage, a complication often treated with platelet transfusion.
MITS is a predictive model of mortality, which incorporates severe complications, such as arterial thrombosis, intracranial hemorrhage, ischemic stroke and myocardial infarction.18 In our population a score ≥ 2 points was associated with a higher probability of death. On the contrary, we did not observe survival differences when applying the French TMA Reference Center Score. A possible explanation for this finding is that our study population is composed of younger patients (median age below 40 years old) than the other registries.
ADAMTS13 activity was carried out in the minority of patients (34 %). The exam still has a low availability in Brazil and is not covered by the Unified Health System (SUS). In our study, quantification of ADAMTS13 activity became available only after 2013. Nevertheless, this test is not yet carried out in our center, therefore, there are still logistical difficulties related to shipping the sample and result availability.
Our study has some limitations, such as the retrospective design, with its inherent drawbacks, and it was carried out in a single center. Also, the sample size is relatively small, which hindered the realization of a multivariate analysis, however, as is known, TTP is a rare disease, so the accumulation of a significant sample size usually requires several years. Besides, we had data available only from patients who underwent at least one session of TPE. It appears reasonable to presume that some patients died before hospital admission and diagnosis. Finally, since the inclusion period of the study spans twenty years, the treatment protocol varied between patients according to the period in which they were treated.
ConclusionsWe describe here the patients’ characteristics and treatment of iTTP in a single center in Brazil. We believe that delayed diagnosis is associated with a worse clinical picture and, perhaps, a higher mortality rate. Besides, we found that more intense hemolysis and manifestations of organ ischemia, such as cardiovascular and neurological, are indicators of death risk.
CRediT authorship contribution statementPatrícia Oliveira Cunha Terra: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Gil Cunha De Santis: Formal analysis, Conceptualization, Supervision, Writing – review & editing. Benedito de Pina Almeida Prado Júnior: Data curation, Supervision. Luciana Correa Oliveira: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing.