
Multiple myeloma (MM) is a malignancy of terminally differentiated B cells. The diagnosis requires the presence of ≥ 10 % clonal plasma cells (PC) in the bone marrow or an extramedullary plasmacytoma associated with at least one secondary organic lesion such as renal failure, anemia, bone lesions, and hypercalcemia or the presence of biomarkers such as ≥60 % bone marrow plasma cells (BMPC), involved to uninvolved free light chain (FLC) ratio ≥100, or the presence of ≥2 marrow lesions on MRI.1
The term extramedullary multiple myeloma (EMM) is characterized by myeloma cells that have gained independence from the bone marrow microenvironment that can occur from the migration of the clone cells outside of the bone marrow but still connected to the bone or by hematogenous spread.2 It is a rare event with an overall incidence of EMM is 13 %: 7 % at diagnosis and 6–20 % at relapse.3 The most common site of involvement is skin when the EMM is present at diagnosis and other tissues such as the liver, pleura, and central nervous system (SNC) at relapse.2,3 The involvement of testicle in the context of multiple myeloma is rare, corresponding to 0.6 % to 2.7 % of extramedullary manifestations.4 Even more rare is bilateral and synchronous testicular plasmacytoma, with only six cases reported in the literature.5–10
Despite novel and effective agents for treating myeloma, patients with the extramedullary disease often exhibit poor response to therapy, suggesting that there may be biological distinctions between intramedullary and extramedullary forms of the disease.11
In this manuscript, we report a case of EMM disease with synchronous bilateral testicle involvement and the cytogenetic abnormalities of the bone marrow and testes samples at relapse.
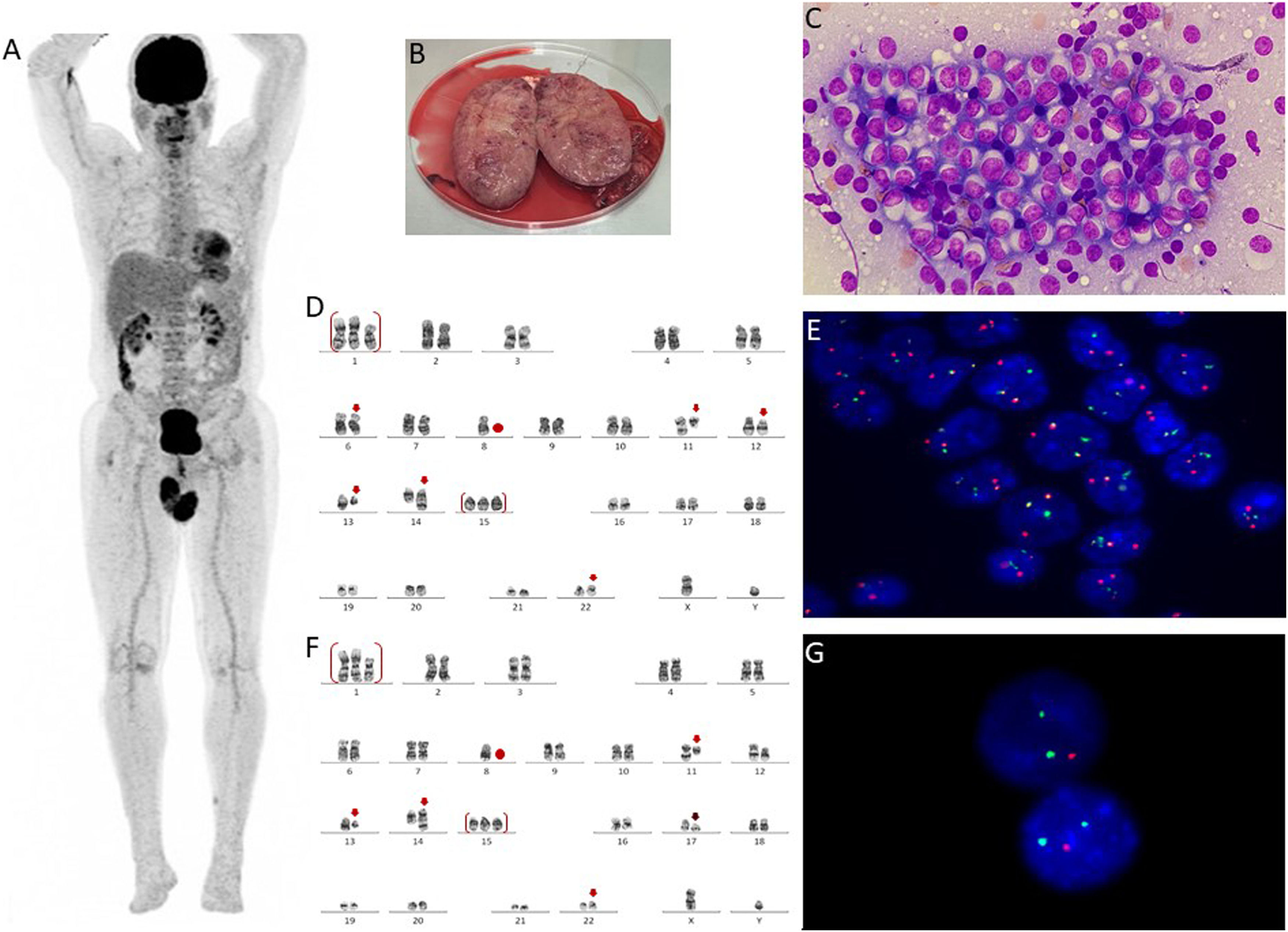
Case reportA 56-year-old male patient was diagnosed with Multiple Myeloma lambda light chain, DS II/ISS II, in June 2018. He had anemia, bone marrow showing 99 % of abnormal plasma cells, normal karyotype, and fluorescence in situ hybridization (FISH) with gain 1q (CKS1B), deletion of part of the long arm of chromosome 13 (RB1) and IGH rearrangement (no FGFR3::IGH and no MAF::IGH fusion). There were no other organ dysfunctions and no lytic bone lesions. He was initially treated with four cycles of VCD (bortezomib, cyclophosphamide, and dexamethasone), followed by autologous bone marrow transplantation (BMT) in 2018 and maintenance with bortezomib. Eleven months after autologous BMT, he relapsed and was treated with DRd (Daratumumab, lenalidomide, and dexamethasone). The second relapse happened two years after the onset of DRd, and the chosen protocol was KCd (carfilzomib, cyclophosphamide, and dexamethasone). He received 12 cycles of KCd and then began biweekly maintenance with carfilzomib and dexamethasone. During the maintenance phase, he presented with a progressive increase in the lambda light chain. About three months after the start of maintenance, he had pain, bilateral testicular edema, and the appearance of a lesion in the left leg. Whole-body PET-CT revealed abnormal uptake in the bilateral testicular region (Figure 1A). Testicular biopsy confirmed testicular plasmacytoma and infiltration by myeloma in a subcutaneous lesion on the left leg. The patient underwent a bilateral orchiectomy (Figure 1B), bone marrow aspirate, and biopsy.
(A) PET-CT shows abnormal testicular uptake (maxim SUV: 10), (B): Significant enlargement of the right testicle (after orchiectomy), (C) Testicle imprint demonstrating high concentration of abnormal plasma cells (Leishman, x 1000), (D) Karyotype of testicular plasma cells showing complex clone: 47,XY,+der(1;6)(q10;p10),del(2)(p13p11.2),der(6)t(6;8)(q21;q22),−8,t(11;14)(q13;q32),del(12)(p13p11.2),del(13)(q12q34),+15,add(22)(p11.2)[20] (GTW banding), (E) Interphase fluorescence in situ hybridization (iFISH) study in testicular plasma cells with IGH::CCND1 dual color, dual fusion translocation probe (Metasystems, Germany) showing two yellow, one green and one red signals suggestive of IGH::CCND1 fusion, (F) Karyotype of bone marrow cells showing complex clone with deletion 17p: 47,XY,+der(1;6)(q10;p10),del(2)(p13p11.2),der(6)t(6;8)(q21;q22),−8,t(11;14)(q13;q32),del(12)(p13p11.2),del(13)(q12q34),+15,del(17)(p11.2),add(22)(p11.2)[7]/46,XY[13], (G) iFISH in CD138 positive cells study with TP53/17cen dual color, deletion probe (Metasystems, Germany) showing two green and one red signals suggestive of deletion TP53.
Testicular imprint showed a high concentration of abnormal plasma cells (Figure 1C), karyotype showed a complex karyotype with t(11;14) (Figure 1D), and FISH studies showed rearrangement CCND1::IGH t(11;14) (Figure 1E), deletion of the RB1 and DLEU1 gene (chromosome 13q), three copies of the CKS1B gene (chromosome 1q) in approximately 94 % of the analyzed nuclei. Bone marrow biopsy identified 5 % of abnormal plasma cells. Cytogenetic analysis of BM revealed the same abnormalities in testicular cells added with deletion 17p in 7 of 20 metaphases analyzed (Figure 1F). Interphasic FISH in CD138 positive cells12 showed the same findings detected in testicular studies in 80 % of nuclei associated with TP53 gene deletion (chromosome 17p) in 42 % of the analyzed nuclei (Figure 1G).
There was no central nervous system infiltration. The patient started with prophylactic intrathecal chemotherapy and was treated with bortezomib, dexamethasone, and venetoclax (dose escalation up to 800 mg /day) for four cycles. After finishing this protocol, we intend to proceed with haploidentical stem cell transplantation.
DiscussionEEM is not a usual primary manifestation, instead, it seems to develop gradually over time with relapses. The occurrence of plasmacytoma involving both testes is rare, and most cases occur asynchronously during relapse. The are only six reports of bilateral and synchronous testicular plasmacytoma: two cases at diagnosis5,10 and four during the relapse.6–9 Our patient also presented the bilateral manifestation during the third relapse.
The risk factors for EMM are not fully elucidated, though, significantly elevated levels of lactate dehydrogenase (LDH), lower hemoglobin and platelet levels, light chain/non-secretory subtypes, more than two lines of treatment and undergoing allogenic steam cell transplantation were reported to be associated with risk for EMM on relapse.3,13,14 The reasons for the discrepancy between autologous and allogenic transplantation are unclear. They may be related to factors such as the selection of more severe patients for allogenic transplantation and the effectiveness of the graft-versus-myeloma effect in the bone marrow.13 The exact reason for the apparent rise in EMM in patients receiving novel agents is not well understood. Currently, there is no evidence that the use of proteasome inhibitors (PIs) and immunomodulatory drugs (IMiDs) in combination leads to quicker onset of EMM.15 On the other hand, studies suggest that the new agents can disturb the bone marrow microenvironment, which could provide an opportunity for a plasma cell clone to escape the bone marrow (BM), which is consistent with the general theory of a clone select in extramedullary myeloma relapse.16 Our patient had some risk factors since the diagnosis such as: anemia, light chain subtype and more than two lines of treatment, including PIs and IMiDs.
Plasma cells originating from extramedullary plasmacytomas (EMPs) typically exhibit an immature or plasmablastic morphology. There have been reports of a transition in secretion pattern from intact immunoglobulin to only free light chains.17 Furthermore, studies have shown that EMM has a higher proliferative index, CD56 is downregulated, and CD44 is upregulated.18 An et al. showed that downregulation of CD56 in MM was significantly correlated with the presence of t(11;14), which appears to aid in the extravasation of plasma cells.19 The bone marrow sample immunophenotyping of our patient showed negativity for CD56 since the diagnosis. CD44 was not evaluated.
Genetic abnormalities associated with MM progress has been described and can be categorized as primary and secondary events. The primary events include translocations involving the immunoglobulin heavy chain (IgH) locus at 14q32, being the t(11;14)(q13q32) the most common, and chromosomal hyperdiploid, which contribute to the immortality of plasma cells. On the other hand, secondary events, which contribute to disease progression, include del(17)(p13), del(13)(q14), MYC overexpression and RAS point mutations.20
Biological and cytogenetic characteristics linked to the extramedullary spread of tumor cells are not well elucidated.1 Since EMM is characterized by the ability of stroma-independent growth and is considered an advanced form of MM, it is presumable that genomic changes will be more common. Indeed, the cytogenetic findings in this case corroborate the idea evidenced by the addition of cytogenetic abnormalities during clinical follow-up and progression of disease.
An interesting aspect about the case was the loss of 17p in the BM sample, while such abnormality was not found in the testes sample, both collected during the relapse. Billecke et al. showed that del(17p13) occurred twice more frequently in the extramedullary manifestation of EMM patients than in the bone marrow (32% vs. 16 %).21 In contrast, Besse et al. did not find any difference in del(17)(p13) incidence between independent BM and extramedullary site samples of EM patients (16% vs. 11 %), and, in paired samples, they observed decreased incidence of del(17)(p13) in extramedullary site samples than in corresponding BM (8% vs. 23 %).22
Another significant aberration found in MM and likewise in EMM is del(13)(q14), and it is often observed in t(11;14) positive MM, which results in haploinsufficiency of the RB tumor suppression gene at 13q14 and consequent disease progression.15,23,24 Recent data show a higher incidence of del(13)(q14) in extramedullary mass compared to BM25 although others are revealing no difference26 or its lower frequency in extramedullary mass.21 Others critical structural abnormalities of chromosomes that appear to be involved in terminal-stage myeloma is 1q gain and deletions of 1p32.27,28 Our patient presented the loss of 13q and 1q gain since the diagnosis as evidenced by FISH and both abnormalities were present in testes samples at relapse.
Other cytogenetic abnormalities not evidenced in our reported case are described. Glitza et al. demonstrated that 8q24.1/c-MYC rearrangement is diverse and can occur as a partial deletion and a typical rearrangement involving different chromosomes partners, all of them setting a high-risk marker, characterized by an increased incidence of plasmablastic morphology and increased incidence of plasma cell leukemia and extramedullary disease.29 Further than MYC, the activation of RAS oncogene seems to mediate the acquisition of an additional growth advantage to the abnormal cell. Rasmussen et al. showed that among six patients who had both intramedullary and extramedullary plasma cells with identical IgH gene sequences, RAS mutations were present only in the plasma cells from tumors in three patients, what suggest that these mutations may play a role in the progression from intramedullary to extramedullary tumors.30 Moreover, the constitutive activation of NF-kb and homozygous deletions of genes encoding inhibitors of the NF-kb pathways such as BIRC2/3 on chromosome 11, TRAF3 on chromosome 14 and CYLD on chromosome 16 seems to be related to stroma independent growth and is often detected at final stage of MM disease.31
Authors’ contributionsConceptualization: MBC and EDRPB. Data curation: MBC, TSD, ACLN, NH. Formal analysis: RMSOS, RKK, DB, MGC and EDRPV. Funding acquisition: RMSOS, RKK, DB, MGC and EDRPV. Project administration: EDRPV. Supervision: EDRPV. Writing: MBC and EDRPV. Investigation: MBC and EDRPV. All authors revised the paper, read and approved the final manuscript.
Data availabilityData sharing does not apply to this article as no datasets were generated or analyzed during the current study.
Ethics statementThe study was approved by the Ethics Committee of Hospital Israelita Albert Einstein (CAAE: 57507322.6.0000.0071). Informed consent was obtained under the Declaration of Helsinki.
We thank our patient for participating in this study. There was no funding specifically for the current work.



![(A) PET-CT shows abnormal testicular uptake (maxim SUV: 10), (B): Significant enlargement of the right testicle (after orchiectomy), (C) Testicle imprint demonstrating high concentration of abnormal plasma cells (Leishman, x 1000), (D) Karyotype of testicular plasma cells showing complex clone: 47,XY,+der(1;6)(q10;p10),del(2)(p13p11.2),der(6)t(6;8)(q21;q22),−8,t(11;14)(q13;q32),del(12)(p13p11.2),del(13)(q12q34),+15,add(22)(p11.2)[20] (GTW banding), (E) Interphase fluorescence in situ hybridization (iFISH) study in testicular plasma cells with IGH::CCND1 dual color, dual fusion translocation probe (Metasystems, Germany) showing two yellow, one green and one red signals suggestive of IGH::CCND1 fusion, (F) Karyotype of bone marrow cells showing complex clone with deletion 17p: 47,XY,+der(1;6)(q10;p10),del(2)(p13p11.2),der(6)t(6;8)(q21;q22),−8,t(11;14)(q13;q32),del(12)(p13p11.2),del(13)(q12q34),+15,del(17)(p11.2),add(22)(p11.2)[7]/46,XY[13], (G) iFISH in CD138 positive cells study with TP53/17cen dual color, deletion probe (Metasystems, Germany) showing two green and one red signals suggestive of deletion TP53. (A) PET-CT shows abnormal testicular uptake (maxim SUV: 10), (B): Significant enlargement of the right testicle (after orchiectomy), (C) Testicle imprint demonstrating high concentration of abnormal plasma cells (Leishman, x 1000), (D) Karyotype of testicular plasma cells showing complex clone: 47,XY,+der(1;6)(q10;p10),del(2)(p13p11.2),der(6)t(6;8)(q21;q22),−8,t(11;14)(q13;q32),del(12)(p13p11.2),del(13)(q12q34),+15,add(22)(p11.2)[20] (GTW banding), (E) Interphase fluorescence in situ hybridization (iFISH) study in testicular plasma cells with IGH::CCND1 dual color, dual fusion translocation probe (Metasystems, Germany) showing two yellow, one green and one red signals suggestive of IGH::CCND1 fusion, (F) Karyotype of bone marrow cells showing complex clone with deletion 17p: 47,XY,+der(1;6)(q10;p10),del(2)(p13p11.2),der(6)t(6;8)(q21;q22),−8,t(11;14)(q13;q32),del(12)(p13p11.2),del(13)(q12q34),+15,del(17)(p11.2),add(22)(p11.2)[7]/46,XY[13], (G) iFISH in CD138 positive cells study with TP53/17cen dual color, deletion probe (Metasystems, Germany) showing two green and one red signals suggestive of deletion TP53.](https://static.elsevier.es/multimedia/25311379/00000046000000S6/v2_202501280810/S2531137924000063/v2_202501280810/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w93OM6WmS6o9DeZl+SVh74uo=)



