Coronavirus disease-2019 (COVID-19) caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) has caused global health crisis. Initially considered a respiratory tract pathogen, it can cause multiple organ dysfunction. It has also been described to predispose to venous and arterial thromboembolism; however, limited published data is available regarding mesenteric thrombosis COVID-19. We conducted a rapid review of current scientific literature available in PubMed to identify cases of AMI in in COVID-19 patients- total of 13 cases were found. We delineated clinical characteristics and outcome in these patients. Clinicians should be aware of the life-threatening situation in COVID-19 patients.
A novel coronavirus termed as SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) has been the causative agent of a pandemic that originated in Wuhan China in December 2019. Coronavirus disease-2019 (COVID-19) can present with a wide variety of complications during infection. For optimal management of these patients, understanding of various systemic manifestations and complications of SARS-CoV2 is vital. Although in COVID-19 respiratory symptoms predominate, both arterial and venous thrombosis can occur with COVID-19. Arterial thrombosis reported so far include stroke, acute limb ischemia, acute mesenteric ischemia and acute coronary syndrome.1–4 Limited literature is available regarding acute mesenteric ischemia (AMI). We did an extensive literature review on COVID-19 associated mesenteric thrombosis.
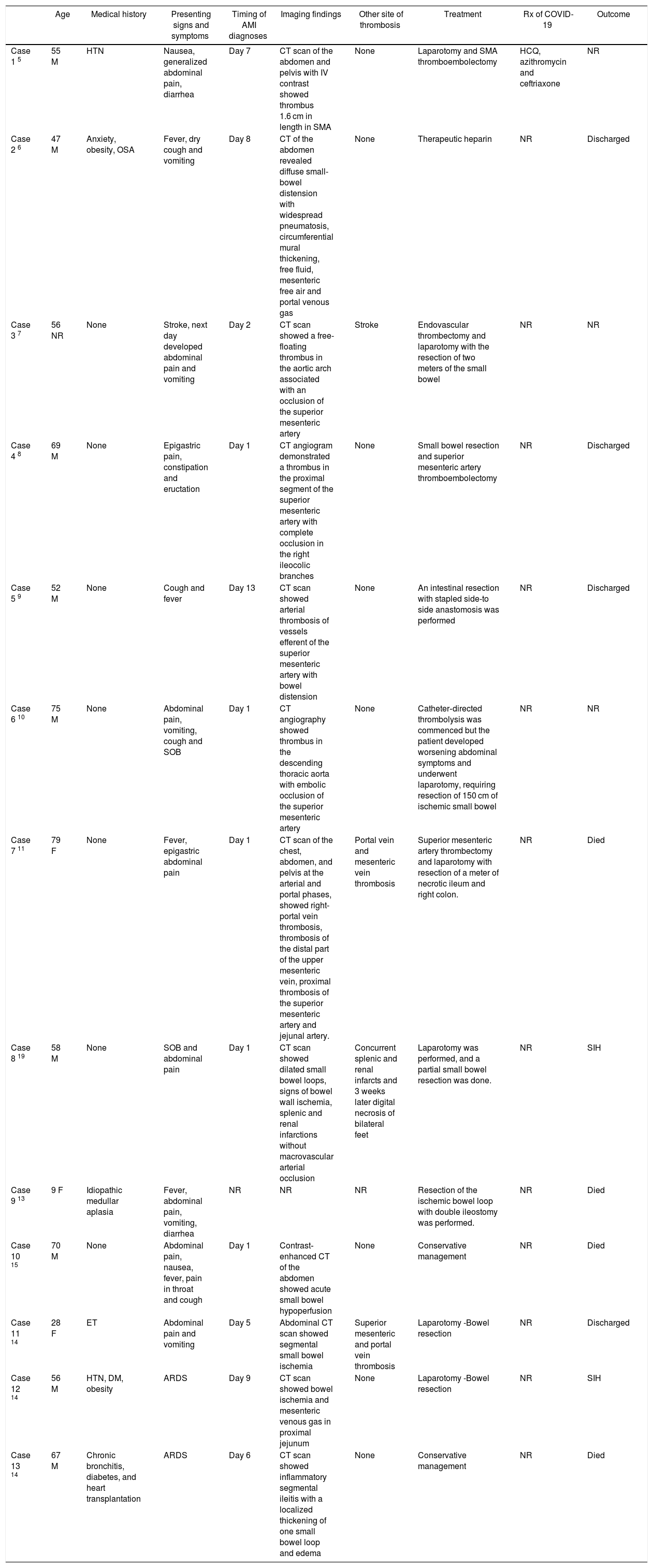
We searched PubMed for this literature review using search terms ‘COVID-19 and mesenteric thrombosis’, ‘COVID-19 and mesenteric ischemia’, and ‘COVID-19 and bowel ischemia’. All the case reports who had COVID-19 associated mesenteric thrombosis so far is reviewed, and relevant data abstracted from these studies in Table 1. COVID-19 diagnosis was made by PCR assay except in one patient it was negative (suspected COVID-19).
Summarizing Clinical characteristics of the COVID-19 patients with AMI.
| Age | Medical history | Presenting signs and symptoms | Timing of AMI diagnoses | Imaging findings | Other site of thrombosis | Treatment | Rx of COVID-19 | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 5 | 55 M | HTN | Nausea, generalized abdominal pain, diarrhea | Day 7 | CT scan of the abdomen and pelvis with IV contrast showed thrombus 1.6 cm in length in SMA | None | Laparotomy and SMA thromboembolectomy | HCQ, azithromycin and ceftriaxone | NR |
| Case 2 6 | 47 M | Anxiety, obesity, OSA | Fever, dry cough and vomiting | Day 8 | CT of the abdomen revealed diffuse small-bowel distension with widespread pneumatosis, circumferential mural thickening, free fluid, mesenteric free air and portal venous gas | None | Therapeutic heparin | NR | Discharged |
| Case 3 7 | 56 NR | None | Stroke, next day developed abdominal pain and vomiting | Day 2 | CT scan showed a free-floating thrombus in the aortic arch associated with an occlusion of the superior mesenteric artery | Stroke | Endovascular thrombectomy and laparotomy with the resection of two meters of the small bowel | NR | NR |
| Case 4 8 | 69 M | None | Epigastric pain, constipation and eructation | Day 1 | CT angiogram demonstrated a thrombus in the proximal segment of the superior mesenteric artery with complete occlusion in the right ileocolic branches | None | Small bowel resection and superior mesenteric artery thromboembolectomy | NR | Discharged |
| Case 5 9 | 52 M | None | Cough and fever | Day 13 | CT scan showed arterial thrombosis of vessels efferent of the superior mesenteric artery with bowel distension | None | An intestinal resection with stapled side-to side anastomosis was performed | NR | Discharged |
| Case 6 10 | 75 M | None | Abdominal pain, vomiting, cough and SOB | Day 1 | CT angiography showed thrombus in the descending thoracic aorta with embolic occlusion of the superior mesenteric artery | None | Catheter‐directed thrombolysis was commenced but the patient developed worsening abdominal symptoms and underwent laparotomy, requiring resection of 150 cm of ischemic small bowel | NR | NR |
| Case 7 11 | 79 F | None | Fever, epigastric abdominal pain | Day 1 | CT scan of the chest, abdomen, and pelvis at the arterial and portal phases, showed right-portal vein thrombosis, thrombosis of the distal part of the upper mesenteric vein, proximal thrombosis of the superior mesenteric artery and jejunal artery. | Portal vein and mesenteric vein thrombosis | Superior mesenteric artery thrombectomy and laparotomy with resection of a meter of necrotic ileum and right colon. | NR | Died |
| Case 8 19 | 58 M | None | SOB and abdominal pain | Day 1 | CT scan showed dilated small bowel loops, signs of bowel wall ischemia, splenic and renal infarctions without macrovascular arterial occlusion | Concurrent splenic and renal infarcts and 3 weeks later digital necrosis of bilateral feet | Laparotomy was performed, and a partial small bowel resection was done. | NR | SIH |
| Case 9 13 | 9 F | Idiopathic medullar aplasia | Fever, abdominal pain, vomiting, diarrhea | NR | NR | NR | Resection of the ischemic bowel loop with double ileostomy was performed. | NR | Died |
| Case 10 15 | 70 M | None | Abdominal pain, nausea, fever, pain in throat and cough | Day 1 | Contrast‐enhanced CT of the abdomen showed acute small bowel hypoperfusion | None | Conservative management | NR | Died |
| Case 11 14 | 28 F | ET | Abdominal pain and vomiting | Day 5 | Abdominal CT scan showed segmental small bowel ischemia | Superior mesenteric and portal vein thrombosis | Laparotomy -Bowel resection | NR | Discharged |
| Case 12 14 | 56 M | HTN, DM, obesity | ARDS | Day 9 | CT scan showed bowel ischemia and mesenteric venous gas in proximal jejunum | None | Laparotomy -Bowel resection | NR | SIH |
| Case 13 14 | 67 M | Chronic bronchitis, diabetes, and heart transplantation | ARDS | Day 6 | CT scan showed inflammatory segmental ileitis with a localized thickening of one small bowel loop and edema | None | Conservative management | NR | Died |
M: male; F: female; NR: not reported; HTN: hypertension; OSA: obstructive sleep apnea; ET: essential thrombocytosis; DM: diabetes; SOB: shortness of breath; ARDS: acute respiratory distress syndrome; CT: computed tomography; SMA: superior mesenteric artery; HCQ: hydroxychloroquine; SIH: Still in hospital (at the time of writing of respective manuscript).
Clinical characteristics of the COVID-19 patients with AMI are summarized in Table 1.5–15 The median age of the patient was 56 years (range 9–79 years). We found total of 13 patients- 9 were male, 3 female and for 1 patient sex was not reported. AMI can occur as a presenting feature or a late complication of COVID-19 during hospitalization (median 7 days). 6 patients had pre-existing comorbidities while 7 patients had none. The pre-existing conditions reported were hypertension, diabetes, obesity, obstructive sleep apnea, anxiety, idiopathic medullar aplasia, chronic bronchitis, essential thrombocytosis, and cardiac transplantation. Presenting symptoms were nausea, vomiting, abdominal pain, diarrhea, fever, cough, shortness of breath, eructation, pain in throat and stroke. The diagnosis of AMI was made by contrast enhanced computed tomography. 4 patients had concurrent thrombosis at other sites – case 3 had stroke, case 7 had portal and mesenteric vein thrombosis, case 8 had splenic and renal infarcts and case 11 had superior mesenteric and portal vein thrombosis. 10 patients had surgery, 2 patients had conservative management and 1 was started on therapeutic anticoagulation with heparin. Out of 13 patients, 4 patients died.
Acute mesenteric ischemia is a rare abdominal emergency and is associated with high rates of morbidity and mortality. Prompt diagnosis requires a high index of suspicion and early contrast computed tomography imaging. The exact pathological mechanism leading to the complication of AMI in COVID-19 is not well understood at present, possibilities include - direct invasion of bowel tissue by the virus given expression of angiotensin converting enzyme 2 on enterocytes, the target receptor for SAR-Cov-2 or viral infection of the endothelial cell leading to diffuse endothelial inflammation or increased procoagulant factors like factor VIII, von Willebrand factor, fibrinogen or virus induced cytokine storm leading to coagulation and fibrinolysis activation.16–18 Additional explanations for the hypercoagulability may be the presence of high numbers of prothrombotic circulating microvesicles which are cytoplasmic microparticles stemming from platelets or monocytes and Neutrophil extracellular traps (NETs) released from activated neutrophils, constitute a mixture of nucleic DNA, histones and nucleosomes.18Treatment of this life-threatening condition includes surgical resection of the necrotic bowel, restoration of blood flow to the ischemic intestine and supportive measure - gastrointestinal decompression, fluid resuscitation, hemodynamic support. Health care providers should have high index of suspicion regarding this life-threatening complication of COVID-19 so that timely intervention can be done.