To investigate, in a large prospective multicenter study, whether 2-[18F]-fluoro-2-deoxy-d-glucose-positron emission tomography is sufficiently accurate to identify clinically important bone marrow involvement by Hodgkin's lymphoma to replace routine bone marrow biopsy in a developing tropical country.
MethodsPatients newly diagnosed with Hodgkin's lymphoma were recruited from six cancer centers in Brazil. All were staged by the results of positron emission tomography/computed tomography that were centrally reviewed and by iliac crest bone marrow biopsy. Patients were classified as having marrow disease if they had lymphoma identified by marrow biopsy histology or had focal 2-[18F]-fluoro-2-deoxy-d-glucose marrow uptake that resolved following chemotherapy.
ResultsA total of 246 participants were recruited from six different centers and 62 (25.2%) were judged to have Hodgkin's lymphoma in the bone marrow. Positron emission tomography and biopsies were concordant in 206 patients (83%). Positron emission tomography correctly identified marrow disease in 59/62 patients (95.1%) and marrow biopsy in 25/62 patients (40.3%). In 22/62 (35.4%) patients, the two techniques were concordant in the diagnosis of marrow involvement. Of the forty discordant results, positron emission tomography found bone marrow involvement in 37 patients, upstaging 22 to stage IV and having an impact on therapeutic decision in nine cases given their reallocation from early to advanced stage. Three false negative positron emission tomography results were obtained with bone marrow biopsy giving positive findings. All three cases were classified as stage IV regardless of bone marrow findings implying no modification in the clinical management. The sensitivity, specificity and accuracy of positron emission tomography for detecting bone marrow disease were 95%, 100% and 98% and for bone marrow biopsy they were 40%, 100% and 84%, respectively.
ConclusionWe conclude that positron emission tomography can replace marrow biopsy in Brazilian patients with Hodgkin's lymphoma without compromising clinical management.
In the last decades, positron emission tomography (PET) with 2-[18F]-fluoro-2-deoxy-d-glucose (FDG) has become the established modality for staging Hodgkin's lymphoma (HL), and international guidelines for its use in lymphomas were harmonized in recent years.1–4
Defining prognosis has been a cornerstone of patient management and trial design in HL. In addition, bone marrow biopsy (BMB) has been standard in HL staging, although it is often performed even when the likelihood of involvement is low. Patients with early-stage disease rarely have bone marrow involvement in the absence of a suggestive FDG-PET finding, and those with advanced-stage disease rarely have bone marrow involvement in the absence of disease-related symptoms or other evidence of advanced-stage disease. Different studies have suggested that if a PET/computed tomography (CT) is performed, the need for BMB is questionable in the evaluation of patients with HL.5–7 However, substantial data is not found in the literature about this issue in patients from developing countries, where disease presentation tends to be more advanced at diagnosis,8 potentially increasing the incidence of marrow involvement. Furthermore, those countries generally have an elevated prevalence of chronic infectious diseases that could theoretically increase the rate of false positive PET results.9–11
This study is part of a Brazilian collaborative project, supported by the Brazilian Society of Nuclear Medicine (SBMN) and the Brazilian Association of Hematology, Hemotherapy and Cellular Therapy (ABHH), to investigate applications of PET/CT in the evaluation of HL patients. Here we evaluate the utility of FDG-PET to detect bone marrow involvement in comparison with BMB as part of initial diagnostic staging of patients with HL in six centers.
MethodsPatients were recruited from six major Brazilian cancer centers: Quanta Diagnóstico e Terapia, Instituto do Câncer do Estado de São Paulo (ICESP), Hospital Sírio Libanês, Hospital Samaritano, Universidade Federal de São Paulo (UNIFESP) and Universidade Estadual de Campinas (Unicamp). The study received Research Ethics Committee approval in all participating institutions and written informed consent was obtained from study participants.
Diagnosis and stagingDiagnosis of de novo HL was made by biopsy of sites of primary lymph node or extranodal disease. Under 18-year-old and pregnant patients were excluded. All patients were staged by unilateral BMB and by FDG-PET/CT scans. Bone marrow from the iliac crest was biopsied and assessed by a local senior hematopathologist.
Whole-body FDG-PET/CT imaging was acquired following standard protocols regarding uptake time (60–90min) after the intravenous administration of 296–444 MBq (8–12mCi) of FDG with a maximum interval of 14 days between BMB and PET scanning. Scanners were calibrated and images scaled for reading according to local protocols. Staging scans were performed prior to treatment.
FDG-PET/CT was classified as negative for bone marrow involvement in the absence of any focal area of increased bone marrow uptake or in the presence of diffuse bone marrow uptake. FDG-PET/CT was considered positive in the presence of focal FDG uptake regardless of diffuse uptake.
Quality controlPET scans were centrally reviewed by two nuclear medicine physicians (JJC, CACPN). For the purpose of this study, all scans having abnormal FDG-uptake in bone marrow recorded in the database were examined to confirm and classify the pattern of marrow involvement.
Categorizing marrow disease and data analysisSince histological examination of all sites is not possible, FDG-PET/CT and BMB results were combined in a reference standard in order to establish the diagnostic accuracy of the methods. Positive concordant findings at FDG-PET and BMB were interpreted as true positives. Concordant negative findings were interpreted as true absence of disease. In discordant cases with focal FDG uptake in marrow, resolution of the abnormal uptake in response to chemotherapy was taken as evidence of true marrow disease, even with no evidence of bone marrow involvement by histological analysis (biopsy findings in these cases were considered not representative, i.e., false negatives). FDG-PET with no apparent marrow disease was considered false negative in patients with a positive BMB.
Statistical analysisThe diagnostic accuracies were analyzed in terms of sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). The Statistical Package for the Social Sciences (SPSS) version 10.0 for Windows (SPSS Inc., Chicago, IL) was used for the statistical analysis.
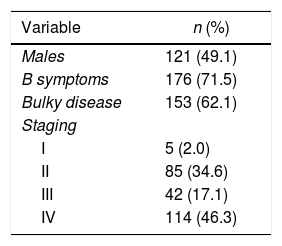
ResultsOverall, 246 patients with complete data available for evaluation were enrolled. One hundred and twenty-one patients were male (49%) with a median age of 33 years (interquartile range: 24–38).
The clinical characteristics of the 246 patients are presented in Table 1. The marrow was considered to be positive for lymphoma in 62 patients (25.2%). Patients were considered to have evidence of marrow disease (true positive), if lymphoma was identified histologically by BMB histology or had focal bone marrow FDG-uptake at PET that resolved following chemotherapy, irrespective of iliac crest histology. Based on this definition, PET correctly identified marrow disease in 59/62 patients (95.1%) and marrow biopsy in 25/62 patients (40.3%).
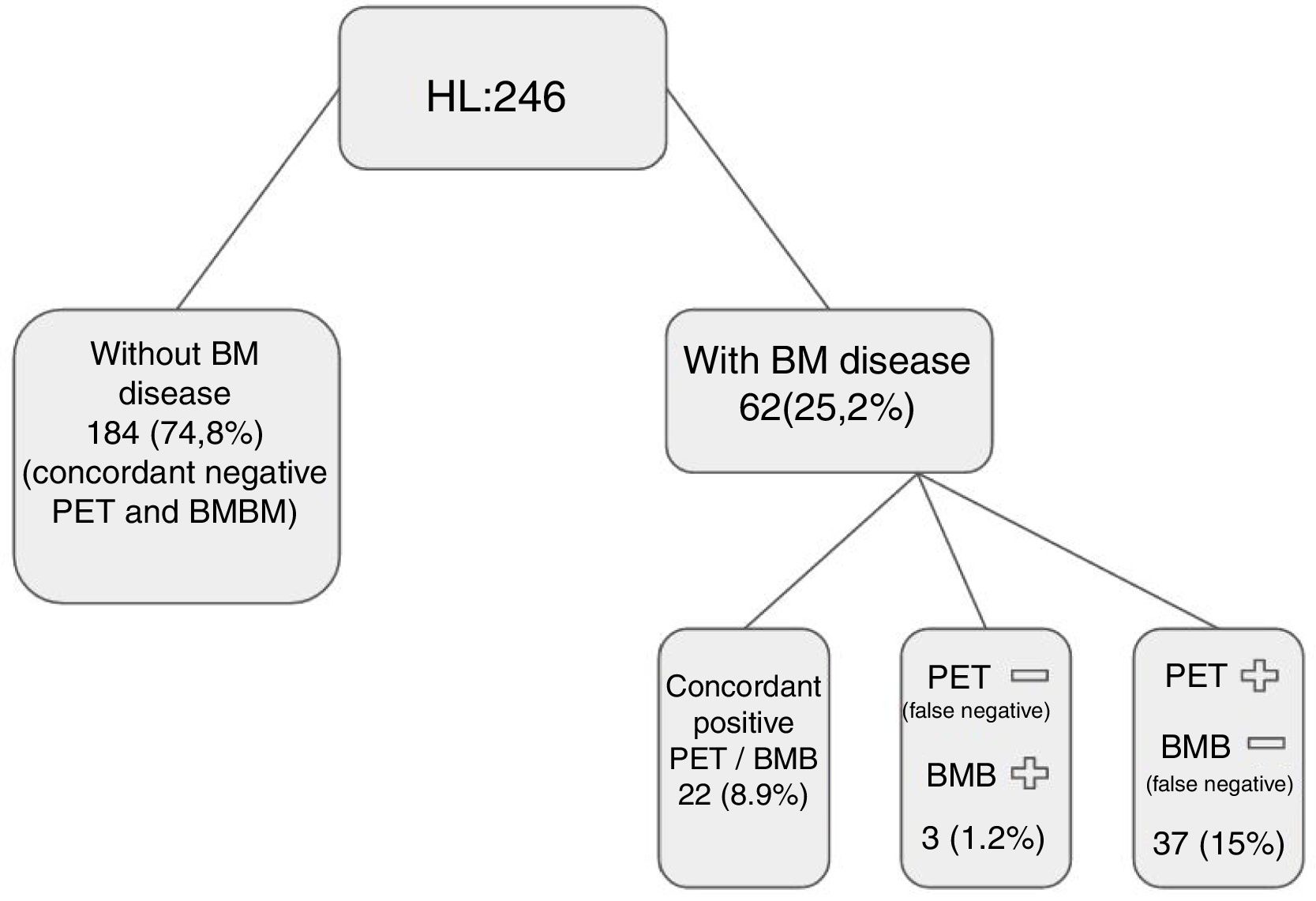
In 22/62 (35.4%) patients, PET scan and marrow biopsy were concordant for the diagnosis of marrow involvement and in 40 patients results were discordant (Figure 1).
positron emission tomography (PET)/computed tomography (CT) versus bone marrow biopsy (BMB): Patients were considered to have evidence of marrow disease (true positive) if lymphoma was identified histologically by BMB histology, or they had focal bone marrow 2-[18F]-fluoro-2-deoxy-d-glucose (FDG)-uptake at PET that resolved following chemotherapy, irrespective of iliac crest histology. Based on this definition, PET correctly identified marrow disease in 59/62 patients (95.1%), marrow biopsy in 25/62 patients (40.3%). In 22/62 (35.4%) patients, PET scan and BMB were concordant in the diagnosis of marrow involvement, and in 40 patients, results were discordant.
All 37 patients with positive PET results and discordant marrow biopsy presented true bone marrow involvement given the resolution of the focal marrow FDG uptake areas following chemotherapy. As a result of PET performance, nine patients were upstaged from stage II to IV (hence, therapeutic decision was changed for this group), 13 were upstaged from stage III to IV, and 11 were already classified as stage IV prior to PET scanning. Three false negative PET results were found (after positive findings in BMB). In all three cases, patients were already classified as stage IV, thus the clinical decision was not changed.
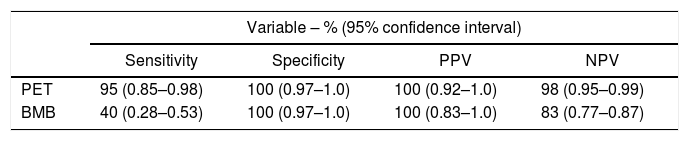
Overall sensitivity, specificity and accuracy of PET for detecting bone marrow disease were 95%, 100% and 98% and for BMB they were 40%, 100% and 84%, respectively (Table 2).
Overall sensitivity, specificity and accuracy comparing positron emission tomography (PET) with bone marrow biopsy (BMB) to detect bone marrow disease.
| Variable – % (95% confidence interval) | ||||
|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | |
| PET | 95 (0.85–0.98) | 100 (0.97–1.0) | 100 (0.92–1.0) | 98 (0.95–0.99) |
| BMB | 40 (0.28–0.53) | 100 (0.97–1.0) | 100 (0.83–1.0) | 83 (0.77–0.87) |
PPV: positive predictive value; NPV: negative predictive value.
This paper reports part of a collaborative project to examine the utility of PET scanning for staging HL in Brazilian patients. The overarching purpose was to assess whether published studies from Western Europe and USA comparing BMB and FDG-PET in Hodgkin's lymphoma5–8,12–14 are generalizable to clinical practice in Brazil given the singularities of our country.
Although the exact rate of patients classified as having advanced stage disease nationwide due to their bone marrow status is unknown, Brazil presents a higher rate of advanced stage HL patients than other populations, thus, it also has a relatively greater number of eligible patients for bone marrow disease assessment, given the higher incidence in advanced stage groups.15 Furthermore, there is still a relatively high incidence of chronic infectious diseases in Brazil, such as tuberculosis, paracoccidioidomycosis, leishmaniasis, histoplasmosis, etc.,9,16–18 that can potentially involve bone marrow and could increase the rate of false-positive results of FDG-PET.19 In addition, the possibility of simultaneous occurrence of lymphoma and specific chronic infections has been reported.11,20
This report focuses on the comparison of PET and BMB to identify bone marrow involvement in patients with newly diagnosed HL.
What we foundBased on information from both PET and BMB, 24% of cases were considered to have bone marrow involvement by HL. As reported by others, PET had greater sensitivity than BMB (PET 98% vs. BMB 40%) with the same specificity (100%). PET identified marrow disease not identified by BMB in 37/62 (59.6%) cases, which translated into upstaging patients to stage IV in 9% of the cohort.
Comparison with other studiesIn a metaanalysis involving five studies regarding PET bone marrow assessments in HL patients (with a total of 191 patients), Pakos et al.21 reached independent estimates for sensitivity and specificity of 76% and 92%, respectively. In a similar, more recent metaanalysis, Adams et al.7 reported pooled estimates of 96.9% and 99.7%, respectively. The results of this study were quite similar with sensitivity and specificity values for bone marrow disease detection by PET of 95% and 100%, respectively. Furthermore, concordant results between BMB and FDG-PET have been reported in the range of 70–80%,21,22 also similar to the findings of this study in Brazil [206/246 (83%) concordant PET and marrow biopsy results]. It is interesting to point out the similar high specificity of FDG-PET in this study as in the mentioned metaanalyses although the prevalence of infectious diseases potentially affecting bone marrow is higher in Brazil than in developed countries. This can be explained by the low incidence of involvement of the bone marrow by tropical infectious diseases; reports of simultaneous lymphoma and tropical infections are anecdotal.11,20 In addition, bone marrow involvement by these infections are more common in immunocompromised individuals, such as those co-infected with human immunodeficiency virus (HIV) or with allograft transplants16; these patients were not included in the current study.
Clinical implicationsThe 1989 Cotswold modification of the Ann Arbor staging criteria abandoned the routine use of invasive procedures such as explorative laparotomy for HL staging, in the wake of improved imaging diagnosis with CT.23 Routine BMB was restricted to patients with CT-assessed stage III/IV disease or stage II disease with adverse ‘unfavorable’ factors, and only then if a positive finding would alter the therapeutic conduct. Although very few patients with early stage HL have a positive BMB24,25 some guidelines still recommend the inclusion of BMB in the routine staging work-up of patients with newly diagnosed HL.26 In the general practice, it is common to perform routine BMB for advanced stage HL due to the higher prevalence of bone marrow involvement, despite the fact that in advanced stage disease a positive BMB is much less likely to have a therapeutic impact than in early stage disease.27,28 The reason to perform BMB is the possible impact on treatment strategy. The reasons not to perform BMB are: (1) it is an unpleasant procedure for the patient, (2) it may delay the initiation of chemotherapy and (3) cost, especially in less developed countries. On the basis of these results BMB would have had an impact on the treatment of only a single patient if staging was performed with CT only, but with PET/CT staging, BMB had no therapeutic impact on any of the 246 patients.29 These results are also in line with a larger study presented recently by El-Galaly et al.13 These authors reviewed the experience of three Danish institutions where both BMB and PET/CT have been performed routinely to stage all patients with HL for several years. The study included 392 patients with HL, including 202 patients with early stage disease and 190 patients with advanced stage disease (according to PET/CT-based staging). Not a single patient with early stage disease had a positive BMB. Four patients with advanced stage disease had positive BMB despite normal FDG uptake in the bones and marrow. However, the treatment strategy was unaffected in all four patients. Therefore, similar to what was observed in this study, BMB was performed on several HL patients without a single therapeutic consequence.
In Brazil, PET was introduced later in comparison to developed countries, mainly due to financial reasons. Regarding the workup for HL, even after promising results from the first international PET studies, it was only after strenuous verification of its local cost-effectiveness that PET started to be incorporated into Brazilian practice.8 The present study corroborates the cost-effectiveness of FDG-PET in HL in Brazil. It demonstrates that, even in a tropical developing country with higher prevalences of advanced HL and infectious diseases, FDG-PET is accurate to identify clinically important bone marrow involvement by HL and can replace routine BMB in most cases. Staging BMB may be restricted to patients whose images or laboratory tests are suggestive of bone marrow involvement and in those with a potential change in treatment.
ConclusionsOur experience with a large multicenter cohort of Brazilian HL patients further confirms the findings of other international studies that FDG-PET is a reliable method to evaluate bone marrow, including in developing countries. When PET/CT analysis is performed during the oncological workup, BMB does not increase the detection of bone marrow involvement with clinical significance and does not affect the therapeutic decision.
Conflicts of interestThe authors declare no conflicts of interest.


![positron emission tomography (PET)/computed tomography (CT) versus bone marrow biopsy (BMB): Patients were considered to have evidence of marrow disease (true positive) if lymphoma was identified histologically by BMB histology, or they had focal bone marrow 2-[18F]-fluoro-2-deoxy-d-glucose (FDG)-uptake at PET that resolved following chemotherapy, irrespective of iliac crest histology. Based on this definition, PET correctly identified marrow disease in 59/62 patients (95.1%), marrow biopsy in 25/62 patients (40.3%). In 22/62 (35.4%) patients, PET scan and BMB were concordant in the diagnosis of marrow involvement, and in 40 patients, results were discordant. positron emission tomography (PET)/computed tomography (CT) versus bone marrow biopsy (BMB): Patients were considered to have evidence of marrow disease (true positive) if lymphoma was identified histologically by BMB histology, or they had focal bone marrow 2-[18F]-fluoro-2-deoxy-d-glucose (FDG)-uptake at PET that resolved following chemotherapy, irrespective of iliac crest histology. Based on this definition, PET correctly identified marrow disease in 59/62 patients (95.1%), marrow biopsy in 25/62 patients (40.3%). In 22/62 (35.4%) patients, PET scan and BMB were concordant in the diagnosis of marrow involvement, and in 40 patients, results were discordant.](https://static.elsevier.es/multimedia/25311379/0000004000000003/v3_201911280709/S2531137918300658/v3_201911280709/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w93OM6WmS6o9DeZl+SVh74uo=)



