The selection of compatible human leukocyte antigen platelets has been associated with improved platelet increments. Therefore, an effective strategy would be the selection of donors who are genetically compatible according to the human leukocyte antigen system. Nonetheless, this is costly as it concerns a highly polymorphic system, which requires a large bank of genotyped donors.
MethodsThis study evaluated the feasibility of virtual crossmatching using EpVix software, which simplifies the identification of compatible donors or donors with acceptable incompatibilities.
ResultsForty-three oncohematological patients were evaluated, in 96 platelet transfusion episodes with 16.3% of the patients being found to be refractory to platelet transfusions. Eight alloimmunized, multitransfused patients were selected to evaluate human leukocyte antigen compatibility against a bank of 336 platelet donors. At least partially compatible donors were found for all patients. The number of compatible donors was found to be inversely proportional to the human leukocyte antigen-panel reactive antibody score of each patient. It was noted that five patients with scores of 15% or less had at least 190 compatible donors; four fully compatible donors were found for two other patients with scores greater than 80% and only one patient (score of 93%) did not have a fully compatible donor. However, for this last patient, 40 donors were partially compatible according to the software.
ConclusionThe results showed the effectiveness of the use of the EpVix tool to identify potential platelet donors for multitransfused and/or alloimmunized patients, even with a small number of human leukocyte antigen genotyped donors available.
Platelet transfusion refractoriness occurs in between 7% and 34% of oncohematological patients.1–3 The causes may be non-immune (accounting for over 80% of the cases) or immune (accounting for about 20% of the cases) due to the presence of antibodies against antigen systems on the surface of the platelets.4 These antibodies may be natural antibodies against the ABO antigens, or alloantibodies, most frequently against the human leukocyte antigen (HLA) class I system (accounting for 80% of the cases5) and less frequently against the human platelet antigen (HPA) system (accounting for from 10% to 20% of the cases) leading to the destruction of transfused platelets.6
Particularly in cases of immune-related refractoriness, it is necessary to select compatible platelet components for transfusion in order to achieve a satisfactory increment and reduce the risk of bleeding and mortality in oncohematological patients, in addition to rationalizing the use of platelet components.7 Although the selection of HLA-matched donors would be the ideal strategy, this approach is costly as it involves a highly polymorphic system. A large bank of genotyped donors would be necessary, which is difficult to establish in a medium-sized or even large-sized hemotherapy service, especially in Brazil, a country with an ethnically diverse population.8
Studies conducted by Duquesnoy et al.9 favored the development of a tool called HLAMatchmaker, which identifies polymorphic sequences of immunogenic amino acid residues (epitopes) at HLA class I and II binding sites (called eplets) accessible to alloantibodies. Histocompatibility reports generated from the patients’ test results enable the identification of compatible donors or donors with acceptable incompatibilities against which no incompatible triplet or eplet is recognized by the recipient's immune system. The HLAMatchmaker algorithm has already successfully identified kidney transplant donors and patients with platelet refractoriness with the algorithm being associated with effective platelet transfusion in the latter case.10
However, HLAMatchmaker analysis requires the creation of temporary files and manual data entry into spreadsheets, which is time consuming and prone to clerical errors.11
Based on HLAMatchmaker, a Brazilian research team has developed a software that was first called EpHLA and later renamed EpVix (platelets.epvix.com.br).12 The platform simplifies the identification of compatible donors or donors with acceptable incompatibilities. The software is easy to use and identifies strongly immunogenic, weakly immunogenic and non-immunogenic epitopes in HLA alleles. It was developed in an Object Pascal programming language and uses the HLAMatchmaker algorithm to generate histocompatibility reports. Its automatic report generation requires the integration of laboratory test result files (HLA typing and anti-HLA antibodies) and public databases (International ImMunoGeneTics Information System – IMGT).13 The efficiency of this software in identifying acceptable incompatibilities in alloimmunized patients has been previously demonstrated in renal transplant patients and is more efficient than the manual use of HLAMatchmaker with regard to accuracy and speed of analysis.12 The use of EpVix software in selecting platelet donors has also been demonstrated recently8; it proved to be a powerful search tool for HLA donors compatible with patients who are refractory to platelet transfusions.
The input data of the HLAMatchmaker algorithm is as follows: HLA genotyping of the donor and patient, cutoff value and patient's alloantibody results by single antigen assay (One Lambda). The algorithm compares the donor's eplets and the patient's HLA molecules, generating a compatibility list. The reports generated by the software allow potential donors to be grouped into three categories: fully compatible HLA, acceptable mismatches, and unacceptable mismatches.12
This study aimed to describe the characteristics of oncohematological patients regarding response to platelet transfusion, associated clinical conditions and the presence of alloantibodies against platelet antigens, as well as to assess the feasibility of virtual crossmatching, in spite of the small bank of HLA donors.
MethodsThis study included 43 over 18-year-old oncohematological patients referred to the hospital de clinicas of the Universidade Federal do Triângulo Mineiro (HC-UFTM) and to the hospital de clinicas of the Universidade Federal de Uberlândia (HC-UFU), and also 336 regular whole blood donors and platelet apheresis donors at the Hemococentro Regional de Uberlândia (HRU) between March 2008 and July 2012. Blood samples were collected from the patients before and between 10 and 60min after transfusion. The study was approved by the Research Ethics Committees of UFTM, UFU and HEMOMINAS (protocol # 1695, 130 and 271, respectively).
Evaluating the response to platelet transfusionPatients’ clinical data were collected through analysis of medical records. All the patients were transfused with leukoreduced cellular components. Post-increment transfusion was calculated using corrected count increment (CCI).14 The CCI was considered unsatisfactory when there were less than 5000 platelets/μL in assessments performed 10–60min after transfusion, and when there were less than 2500 platelets/μL in evaluations performed after 18–24h.15 Patients who presented two or more consecutive unsatisfactory CCIs were considered refractory to transfusion.
Antibody identificationNon-specific detection of anti-platelet antibodies was performed using the platelet immunofluorescence test (PIFT). The technique was run by flow cytometry using F(ab’) 2-Goat anti-Rabbit IgG (FITC) (Invitrogen, San Diego, CA, USA). Serum antibody testing of 24 healthy male subjects with no history of previous exposure to transfusions or transplants was performed to characterize fluorescence in a standard curve. After checking the homogeneity and normality of the results, the mean fluorescence and the standard deviation (SD) of these samples were determined. The cutoff intervals to characterize results were defined as follows: values lower than R1 (mean±1 SD of mean) were considered negative results; values between R1 and R2 (mean+2SD of mean) inconclusive, and values higher than R2, positive.
The identification of specific antibodies against HLA class I antigens was achieved by panel reactive antibody assay (PRA) using the Luminex platform LABScreen® (LS1PRA, One Lambda, Canoga Park, CA, USA) and single antigen assay (One Lambda, Canoga Park, CA, USA) according to the manufacturer's instructions. A mean fluorescence intensity (MFI) of 500 was considered the cutoff value. Micro SSP™ DNA typing plates (Biometrix, One Lambda, Canoga Park, CA, USA) were used for HLA class I genotyping; they were coated with a specific oligonucleotide primer sequence to amplify HLA alleles and the human β-globin gene by polymerase chain reaction (PCR).
Virtual cross-reactivityThis study aimed to analyze the possibility of finding compatible donors using the EpVix software (LIB & UFPI©, Teresina, PI, Brazil) to identify fully or partially compatible platelets. For this, eight alloimmunized patients were randomly selected for evaluation by virtual crossmatching against a group of 336 HLA-phenotyped platelet donors. Four of these patients presented satisfactory CCI and four had unsatisfactory CCI.
Virtual crossmatching was carried out using EpVix, which is a free, web-based application developed for use on the Internet. Briefly, in a first step, the results of HLA genotyping of the patient were inserted in the program. In the next step, the results of a single antigen assay (One Lambda) were uploaded to the software. Patient's eplets (self-eplets) were identified and the remaining eplets (non-self-eplets) were then counted and categorized either as reactive or non-reactive based on the mean fluorescence intensity (MFI) cutoff value (defined as 500 according to manufacturer's directions). Non-reactive eplets were those presenting HLA alleles with an MFI value lower than the cutoff value using the panel. Reactive eplets were those appearing only in HLA alleles which had MFI values higher than the cutoff value (for detailed software information, please see Refs. [8,16]). The last step was to perform the virtual HLA Class I crossmatch against the group of donors already uploaded to the software. Donors were considered partially compatible when there was at least one non-reactive eplet, and compatible when there were no non-reactive incompatible eplets.
Statistical analysisThe chi-square test or Fisher's exact test were used for the evaluation of categorical variables. The significance level for rejecting the null hypothesis was 5% (p-value <0.05).
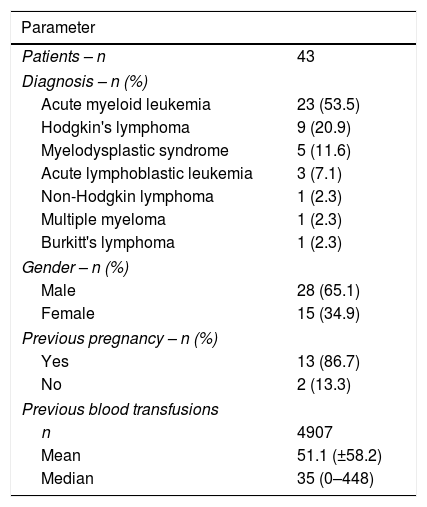
ResultsThis study evaluated 43 oncohematological patients, 28 men and 15 women. The number of transfusions ranged between one and 448 (median of 35 transfusions) and the patients were evaluated during 96 episodes of platelet concentrate transfusions. Patients’ characteristics are described in Table 1.
Characterization of the patients regarding the diagnosis, gender, transfusion history and gestational history.
| Parameter | |
|---|---|
| Patients – n | 43 |
| Diagnosis – n (%) | |
| Acute myeloid leukemia | 23 (53.5) |
| Hodgkin's lymphoma | 9 (20.9) |
| Myelodysplastic syndrome | 5 (11.6) |
| Acute lymphoblastic leukemia | 3 (7.1) |
| Non-Hodgkin lymphoma | 1 (2.3) |
| Multiple myeloma | 1 (2.3) |
| Burkitt's lymphoma | 1 (2.3) |
| Gender – n (%) | |
| Male | 28 (65.1) |
| Female | 15 (34.9) |
| Previous pregnancy – n (%) | |
| Yes | 13 (86.7) |
| No | 2 (13.3) |
| Previous blood transfusions | |
| n | 4907 |
| Mean | 51.1 (±58.2) |
| Median | 35 (0–448) |
It was noted that 21 (48.8%) of the 43 patients had unsatisfactory increments in at least one transfusion episode, platelet transfusion refractoriness was confirmed in seven (16.3%) patients, and 35 (36.5%) of the 96 episodes evaluated presented unsatisfactory post-transfusion increments.
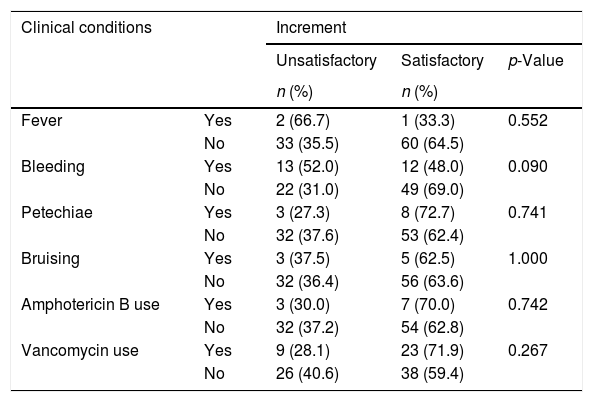
Regarding gender, the percentage of unsatisfactory increments was similar between males (35.7%; n=20) and females 15 (37.5%; n=15) (p-value=1.000). Among the 35 women with history of pregnancy, 42.9% had unsatisfactory increments in comparison with 0% of five women without history of pregnancies, but this difference was not statistically significant (p-value=0.137). There were also no significant differences in platelet increments regarding the clinical status of the patients (Table 2).
Platelet increment assessment regarding clinical conditions of 43 patients and 96 transfusion episodes.
| Clinical conditions | Increment | |||
|---|---|---|---|---|
| Unsatisfactory | Satisfactory | p-Value | ||
| n (%) | n (%) | |||
| Fever | Yes | 2 (66.7) | 1 (33.3) | 0.552 |
| No | 33 (35.5) | 60 (64.5) | ||
| Bleeding | Yes | 13 (52.0) | 12 (48.0) | 0.090 |
| No | 22 (31.0) | 49 (69.0) | ||
| Petechiae | Yes | 3 (27.3) | 8 (72.7) | 0.741 |
| No | 32 (37.6) | 53 (62.4) | ||
| Bruising | Yes | 3 (37.5) | 5 (62.5) | 1.000 |
| No | 32 (36.4) | 56 (63.6) | ||
| Amphotericin B use | Yes | 3 (30.0) | 7 (70.0) | 0.742 |
| No | 32 (37.2) | 54 (62.8) | ||
| Vancomycin use | Yes | 9 (28.1) | 23 (71.9) | 0.267 |
| No | 26 (40.6) | 38 (59.4) | ||
Statistically significant when p-value <0.05.
The PIFT, performed in 41 patients, was positive in 24 (58.5%) individuals, inconclusive in four (9.8%), and negative in 13 (31.7%). The test was not performed in two patients, as their samples were insufficient to be tested. Fifteen (62.5%) patients with positive PIFT were male and nine (37.5%) were female. All these patients (100%) had been previously transfused and eight women (88.9%) had history of pregnancy. Statistical differences were not found on comparing these characteristics between patients with positive and negative PIFT.
The HLA-PRA test was performed in 22 of 24 patients with positive PIFT (the test was not performed in two patients due to methodological problems). The panel-reactive antibody levels ranged from 2% to 96%. In four patients (9.3%) the reactivity was higher than 70%. Moreover, three of these four patients had unsatisfactory increments at the time the analysis was carried out.
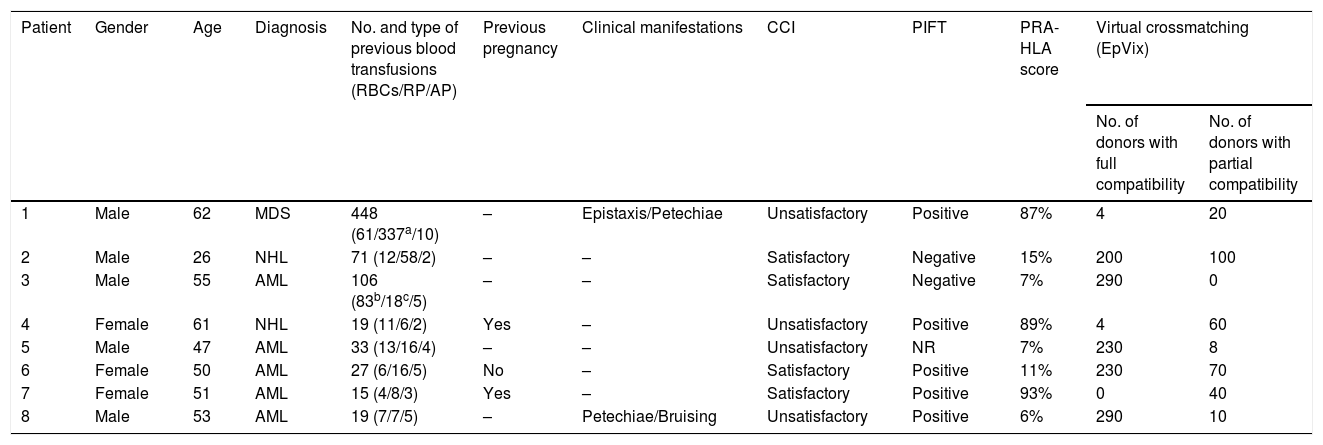
Eight multitransfused patients (4–448 transfusions), four with unsatisfactory CCI and four with satisfactory CCI, were randomly selected to identify compatible donors within a group of 336 HLA-phenotyped donors, in order to evaluate the applicability and effectiveness of the EpVix software.
The results of the virtual crossmatching are showed in Table 3. At least partially compatible donors were found for all eight patients; fully compatible donors were identified for seven of the patients. The number of compatible donors was inversely proportional to the PRA reactivity rate of each patient. Two hundred and ninety and 230 donors with full compatibility were encountered for Patients 3 and 5, respectively (HLA-PRA score of 7% for both). For Patient 8, with a HLA-PRA score of 6%, 290 possible donors were found. Patients 6 and 2 (HLA-PRA scores of 11% and 15%, respectively) were fully compatible with 230 and 200 donors. Patients 1 and 4 had HLA-PRA scores of 87% and 89%, respectively, and four fully compatible donors were found for each. For Patient 7 (HLA-PRA score of 93%) no donors with non-reactive eplets could be found; however, 40 donors were identified by the software as partially compatible.
Evaluation of eight patients regarding clinical and immunological characteristics and analysis of HLA compatibility using the EpVix software with a database of 336 platelet donors.
| Patient | Gender | Age | Diagnosis | No. and type of previous blood transfusions (RBCs/RP/AP) | Previous pregnancy | Clinical manifestations | CCI | PIFT | PRA-HLA score | Virtual crossmatching (EpVix) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of donors with full compatibility | No. of donors with partial compatibility | ||||||||||
| 1 | Male | 62 | MDS | 448 (61/337a/10) | – | Epistaxis/Petechiae | Unsatisfactory | Positive | 87% | 4 | 20 |
| 2 | Male | 26 | NHL | 71 (12/58/2) | – | – | Satisfactory | Negative | 15% | 200 | 100 |
| 3 | Male | 55 | AML | 106 (83b/18c/5) | – | – | Satisfactory | Negative | 7% | 290 | 0 |
| 4 | Female | 61 | NHL | 19 (11/6/2) | Yes | – | Unsatisfactory | Positive | 89% | 4 | 60 |
| 5 | Male | 47 | AML | 33 (13/16/4) | – | – | Unsatisfactory | NR | 7% | 230 | 8 |
| 6 | Female | 50 | AML | 27 (6/16/5) | No | – | Satisfactory | Positive | 11% | 230 | 70 |
| 7 | Female | 51 | AML | 15 (4/8/3) | Yes | – | Satisfactory | Positive | 93% | 0 | 40 |
| 8 | Male | 53 | AML | 19 (7/7/5) | – | Petechiae/Bruising | Unsatisfactory | Positive | 6% | 290 | 10 |
RBCs: packed red blood cells; RP: random platelet concentrate; AP: Apheresis platelet concentrate; CCI: corrected count increment; PIFT: platelet immunofluorescence test; PRA-HLA: panel reactive antibody test; AML: acute myeloid leukemia; NHL: non-Hodgkin lymphoma; MDS: myelodysplastic syndrome.
Of the patients analyzed in this study, 16.3% had platelet refractoriness. It is known that the response to platelet transfusion is influenced by three important factors: quality of platelet concentrate (PC), clinical conditions of the patient and immunological factors. Several studies have demonstrated that factors inherent to the patient are the main determinants of transfusion efficacy, without regard to factors connected with the quality of platelet components.1,2,14,17,18 In this study, 30% of alloimmunized patients developed refractoriness to platelet transfusions.
Most (86.7%) of the women had history of pregnancy and this group presented a higher frequency of unsatisfactory increments and positive PIFT. Doughty et al. reported that being female has a negative influence on the efficacy of transfusion, because most women have a history of pregnancy; according to the TRAP Study Group, women with two or more pregnancies have a greater risk of developing refractoriness.1,19
As for the clinical conditions of the patients, petechiae and bruises apparently did not influence the increment in platelets, whereas bleeding was more frequent in individuals with unsatisfactory increments even though this was not statistically significant. Similar results with significant differences have been demonstrated by other studies.2,14,17,20 Regarding the use of medications, no higher frequency of unsatisfactory increments was found among individuals using amphotericin B and vancomycin, unlike reports from other studies.17,21 Furthermore, this study did not find statistical differences in the frequency of alloimmunization regarding gender, previous transfusions or previous pregnancy. This was certainly due to the small number of cases evaluated.
Platelet transfusion in patients with bleeding has a therapeutic role and it is expected that the transfusion helps to stop the bleeding and obtain satisfactory increments. Failures in this procedure may occur due to characteristics of the underlying disease and the clinical conditions associated with it, immune mechanisms (such as alloimmunization), fever, degree of bleeding, and other conditions which hinder the hemostatic function.22
Before the use of leukoreduced blood components, 45–70% of chronically transfused patients developed antibodies against HLA class I antigens.23 The TRAP study showed that after leukoreduction of blood products, there was a significant reduction in the HLA alloimmunization rate (45–17%).1 However, leukodepletion did not promote a significant reduction of HPA alloimmunization.24 Nevertheless, the importance of HPA alloimmunization is controversial.25 The use of leukoreduced blood components has been an important alternative in reducing alloimmunization, but the best alternative for achieving a better platelet increment is the use of HLA-compatible platelets.26
Upon considering the conditions related to prior sensitization to transfused platelet antigens, most patients had been exposed to one type of blood component, and it was observed that the higher the number of previous transfusions, the higher the risk of sensitization.27 Furthermore, when a sensitized individual, who is a strong immune responder is exposed to incompatible platelet concentrates, this patient is more likely to present transfusion reactions and inadequate responses to transfusion.17,18 The frequency of alloimmunized patients in this study was 58.5%. Other studies with oncohematological patients have shown frequencies ranging from 7% to 66%.2,20,27,28 Alloantibodies may become undetectable in some individuals, even when exposed to a specific antigen, whereas in other patients, the alloantibodies persist for years after the last transfusion.29
It should be noted that in the present study all patients had received leukoreduced components, and even then, a high percentage of alloimmunized patients were found. This would be explained by the fact that, despite the use of leukoreduced platelet concentrates, bedside-filtered randomized platelets were used in most cases, increasing antigen exposure, alloimmunization and refractoriness.
In this study, only 47.2% of individuals with unsatisfactory increments had positive PIFT. However, this frequency was significantly higher than in individuals with negative PIFT (p-value <0.05). Among the four patients with positive HLA-PRA, three (75%) had unsatisfactory CCI. These results reinforce the idea that the alloantibodies produced may favor the early onset of platelet removal from the bloodstream, thus impairing the response to transfusion.17
Nonetheless, the presence of alloantibodies is not synonymous with unsatisfactory increments.30 This was observed in the current study, in which only about 30% of alloimmunized patients developed refractoriness to platelet transfusions. Among the 43 patients included in this study and the 96 episodes evaluated, seven had platelet refractoriness (16.3%). The frequency observed was similar to the frequencies reported in the literature, ranging from 7% to 34%.1,2,17,20,31
Upon assessing the effectiveness of the EpVix software in identifying compatible platelets in eight patients – in a context in which approximately half of the multitransfused patients were alloimmunized and presented refractoriness – it was observed that approximately two-thirds of the donors were compatible for five individuals, four donors were compatible with two other patients, and only one had no fully compatible donor. However, 40 donors with acceptable mismatches were found by the software for this latter patient. Thus, in the absence of other possibilities, this individual could receive platelet transfusion with reasonable chances of satisfactory increments. Nevertheless, in this case, further confirmatory serological tests are recommended, such as flow cytometry crossmatching and a lymphocyte toxicity assay. EpVix uses PRA data and converts them into epitope language. In more alloimmunized patients (with higher PRA reactivity), more reactive epitopes will be identified and fewer compatible donors will be found.
The use of HLA-compatible products in refractory patients results in greater increments one hour after the transfusion, in contrast with the use of randomly obtained platelet concentrates. Therefore, the use of HLA-compatible platelets in patients with alloimmune refractoriness or patients suspected of having such refractoriness is strongly recommended in transfusion medicine. However, structuring a group of HLA donors in order to find identical donors is time-consuming and labor-intensive, as it requires a large amount of genotyped donors as well as considerable investment.7 On the other hand, the identification of donors with acceptable mismatches based on patient antibody reactivity has proven to be an effective alternative approach.32
Virtual crossmatching for platelet transfusion is a new methodology of employing the EpVix tool that has proven to be effective, feasible and fast for identifying HLA platelet donors compatible with alloimmunized patients.8 This study confirms the efficacy of the method, showing that even in multitransfused and refractory patients, with a small number of available HLA genotyped donors, it was possible to identify compatible or partially compatible donors, improving both transfusion efficiency and management.
Conflicts of interestThe authors declare no conflicts of interest.
FundingThis work was supported by Fundação HEMOMINAS, Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Universidade Federal do Triângulo Mineiro (UFTM).
AuthorshipMillena Gomes Ferreira, Aline Aparecida Ferreira and Carolina Bonet Bub performed the research, Fernando Antônio Vinhal dos Santos and Adilson Botelho Filho, Fernanda Bernadelli De Vito and Sheila Soares analyzed the data and wrote the paper and Millena Gomes Ferreira and Helio Moraes-Souza designed the research study, analyzed the data and wrote the paper.