Myelodysplastic syndrome (MDS)-myeloproliferative neoplasm (MPN) with ringed sideroblasts and thrombocytosis (MDS/MPN-RS-T) is a rare entity categorized under the myelodysplastic-myeloproliferative group (MDS/MPN) in the recent 2016 World Health Organization (WHO) update on the classification of myeloid neoplasms.1 Initially named as refractory anemia with ringed sideroblasts associated with marked thrombocytosis (RARS-T), it was a provisional entity under MDS/MPN in the 2008 WHO classification of hematopoietic tumors. Patients with this condition have clinico-morphological features of MDS (RARS) along with marked thrombocytosis and abnormal megakaryocytes similar to those seen in breakpoint cluster region-Abelson murine leukemia-1 (BCR ABL1)-negative MPN.
Case reportWe report the case of a 60-year-old non-alcoholic male with complaints of generalized weakness, progressive pallor and abdominal discomfort over one year. He had no history of bleeding from any site, passage of worms, bone pain, loss of appetite, weight loss, jaundice, persistent fever, respiratory or genitourinary complaints, or recent blood transfusions. On general examination, he was afebrile with the presence of pallor. No lymph nodes were palpable. On abdominal examination, he had hepatomegaly (5cm below costal margin) and massive splenomegaly. An examination of the respiratory and cardiovascular systems was unremarkable. The patient's diagnostic work up included a complete blood count with bone marrow examination. Other tests were performed to rule out nutritional deficiencies (iron and vitamin B12 and folic acid), renal or hepatic disorders, loss of blood in stool and viral infections.
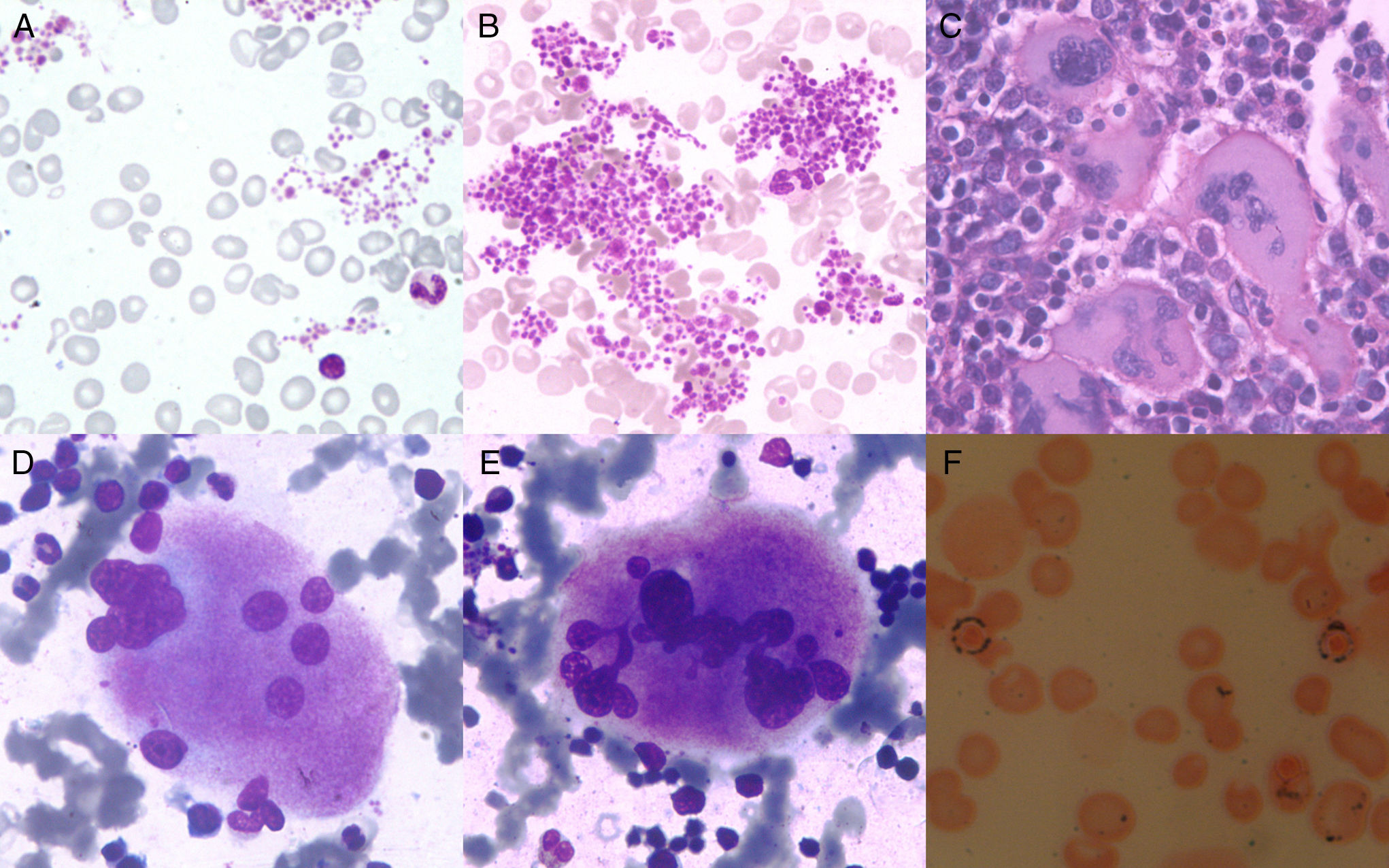
Routine complete blood count revealed a hemoglobin level of 5.6g/dL, red blood cell (RBC) count 1.66×106/μL, mean corpuscular volume 119fL, mean corpuscular hemoglobin 33.8pg, mean corpuscular hemoglobin concentration 28.4g/dL, RBC distribution width 22.9, leukocyte count 13.6×103μL−1, DLC-P69 L25 E5 M1, platelet count 1500×109L–1 and mean platelet volume (MPV) 13.4fL. By peripheral blood smear, the RBCs showed anisocytosis and macrocytosis. Plenty of large platelet clumps were seen with marked platelet anisocytosis (Figure 1A and B). His iron profile, serum vitamin B12 levels and serum folate levels were normal. Liver function tests (serum bilirubin, serum alkaline phosphatase, serum aminotransferases) and kidney function tests (serum urea and serum creatinine) were within the normal ranges. Serology for viral markers [hepatitis C virus, human immunodeficiency virus and hepatitis B virus (HBsAg)], and stool tests for occult blood were negative. Routine urine microscopy was normal. Bone marrow trephine sections were hypercellular (cellularity: 90%) with disorganized marrow architecture. Megakaryocytes were increased in numbers with random distribution in the interstitium and in occasional loose clusters. Few paratrabecular megakaryocytes were also visualized. The megakaryocytes seen were large giant forms with hyper-lobulated nuclei (stag-horn forms); occasional dysplastic forms with disjointed lobes were also seen (Figure 1C–E). Erythroid precursors were increased with clustering in places. Erythroid precursors showed megaloblastoid changes. The myeloid to erythroid ratio was 1:4. Granulocytic precursors had normal morphology with maturation. Bone marrow iron stain revealed 35% ringed sideroblasts (Figure 1F). Cytogenetic analysis of 20 metaphase spreads revealed a normal karyotype. Fluorescent in situ hybridization (FISH) for the BCR-ABL fusion and reverse transcription polymerase chain reaction (RT-PCR) for the JAK 2V617F mutation were negative. Considering the megakaryocyte morphology and proliferation of erythroid precursors with 35% ringed sideroblasts, a diagnosis of Myelodysplastic/myeloproliferative neoplasm with ringed sideroblasts and thrombocytosis (MDS/MPN-RS-T) was reached. In this case, the differential of RARS with reactive thrombocytosis was ruled out by searching for any underlying condition that might have led to secondary thrombocytosis. Essential Thrombocythemia (ET) was ruled out by the presence of 35% ringed sideroblasts along with the prominent erythroid proliferation. Megakaryocytes with bizarre morphology as seen in myelofibrosis were not found, ruling out this differential diagnosis.
(A–F) A and B: Peripheral Blood Smear (leishman 400×): Macrocytosis (2+) of red blood cells and large platelet clumps; C: Bone marrow biopsy (hematoxylin and eosin 100×): Large megakaryocytes with hyperlobulated nuclei; D and E: Bone marrow aspirate (leishman 400×): Megakaryocytes with Stag-horn nuclei, dysplastic form with disjointed nuclei; F: Perl's stain oil immersion: ringed sideroblasts.
The diagnostic criteria for MDS/MPN-RS-T include thrombocytosis (platelet count ≥450×109/L) associated with refractory anemia. Bone marrow has megakaryocytic hyperplasia and morphologically abnormal megakaryocytes resembling those of ET or primary myelofibrosis (PMF). There was evidence of dyserythropoiesis along with ring sideroblasts (more than 15% of erythroid precursors). In addition, there was no increase in blasts in the peripheral blood (<1%) or bone marrow (<5%). Cases with specific chromosomal abnormalities such as isolated del(5q), inv(3)(q21q26.2), t(3;3)(q21;q26.2), BCR-ABL1 fusion, rearrangement of PDGFRA, PDGFRB or FGFR1 or PCMI-JAK2 must be excluded. Furthermore, there should be no preceding history of MPN, MDS (except MDS-RS) or other MDS/MPN. Mutations in the spliceosome gene SF3B1 have been added as diagnostic criteria, however they are not essential.1
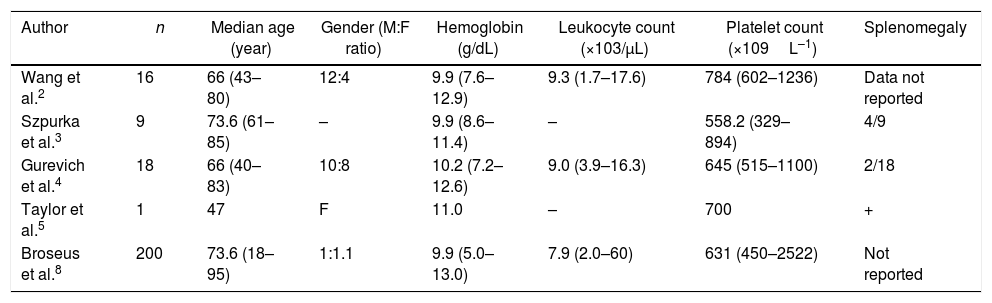
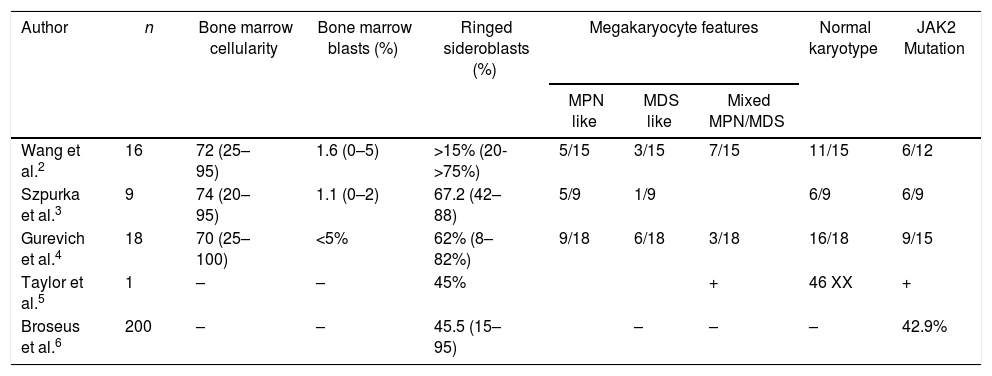
The clinical and pathological features of reported cases of MDS/MPN-RS-T are summarized in Tables 1 and 2.2–6
Clinical characteristics of myelodysplastic syndrome-myeloproliferative neoplasm with ringed sideroblasts and thrombocytosis patients.
| Author | n | Median age (year) | Gender (M:F ratio) | Hemoglobin (g/dL) | Leukocyte count (×103/μL) | Platelet count (×109L–1) | Splenomegaly |
|---|---|---|---|---|---|---|---|
| Wang et al.2 | 16 | 66 (43–80) | 12:4 | 9.9 (7.6–12.9) | 9.3 (1.7–17.6) | 784 (602–1236) | Data not reported |
| Szpurka et al.3 | 9 | 73.6 (61–85) | – | 9.9 (8.6–11.4) | – | 558.2 (329–894) | 4/9 |
| Gurevich et al.4 | 18 | 66 (40–83) | 10:8 | 10.2 (7.2–12.6) | 9.0 (3.9–16.3) | 645 (515–1100) | 2/18 |
| Taylor et al.5 | 1 | 47 | F | 11.0 | – | 700 | + |
| Broseus et al.8 | 200 | 73.6 (18–95) | 1:1.1 | 9.9 (5.0–13.0) | 7.9 (2.0–60) | 631 (450–2522) | Not reported |
Pathological characteristics of myelodysplastic syndrome-myeloproliferative neoplasm with ringed sideroblasts and thrombocytosis cases.
| Author | n | Bone marrow cellularity | Bone marrow blasts (%) | Ringed sideroblasts (%) | Megakaryocyte features | Normal karyotype | JAK2 Mutation | ||
|---|---|---|---|---|---|---|---|---|---|
| MPN like | MDS like | Mixed MPN/MDS | |||||||
| Wang et al.2 | 16 | 72 (25–95) | 1.6 (0–5) | >15% (20->75%) | 5/15 | 3/15 | 7/15 | 11/15 | 6/12 |
| Szpurka et al.3 | 9 | 74 (20–95) | 1.1 (0–2) | 67.2 (42–88) | 5/9 | 1/9 | 6/9 | 6/9 | |
| Gurevich et al.4 | 18 | 70 (25–100) | <5% | 62% (8–82%) | 9/18 | 6/18 | 3/18 | 16/18 | 9/15 |
| Taylor et al.5 | 1 | – | – | 45% | + | 46 XX | + | ||
| Broseus et al.6 | 200 | – | – | 45.5 (15–95) | – | – | – | 42.9% | |
The SF3B1 mutation is often found in association with the JAK2V617F mutation (in >60% of cases), and much less commonly in association with the CALR or MPL W515 mutation (<10% of cases).1,7 The occurrence of these mutations with SF3B1 provides for an overlap of myeloproliferative and myelodysplastic features in this entity.
The presence of the JAK2 V617F mutation has been reported to be associated with MPN like features and a higher platelet count.6,8 It is also associated with favorable prognosis.9 Unmutated JAK2 cases usually have MDS like features with a platelet count of less than 6 linear attenuation coefficient (lac)/μL.8 Patient age, and JAK2V617 and SF3B1 mutations have been reported as independent prognostic factors.1
The current case differs from the previously reported cases in having massive splenomegaly and a higher platelet count despite negativity for the JAK2 V617F mutation. Other mutations like SF3B1, CALR, MPLW515K were however not evaluated for.
The diagnostic differentiation of MDS/MPN-RS-T from RARS and ET cases has prognostic significance. The prognosis of MDS/MPN-RS-T is better than RARS but poor when compared to ET or cases with isolated del 5q.2,6
ConclusionsMDS/MPN-RS-T is a rare disease entity however it should be considered in patients with thrombocytosis and megakaryocytic hyperplasia comprising of giant forms and an increased proliferation of erythroid precursors. Iron staining for ringed sideroblasts is a must in these cases.
The diagnostic differentiation of ET and myelodysplasia with ring sideroblasts is prognostically relevant.
Conflict of interestThe authors declare no conflicts of interest.