Chimeric antigen receptor T-cells (CAR-T cells) are a new modality of oncological treatment which has demonstrated impressive response in refractory or relapsed diseases, such as acute lymphoblastic leukemia (ALL), lymphomas, and myeloma but is also associated with unique and potentially life-threatening toxicities. The most common adverse events (AEs) include cytokine release syndrome (CRS), neurological toxicities, such as the immune effector cell-associated neurotoxicity syndrome (ICANS), cytopenias, infections, and hypogammaglobulinemia. These may be severe and require admission of the patient to an intensive care unit. However, these AEs are manageable when recognized early and treated by a duly trained team. The objective of this article is to report a consensus compiled by specialists in the fields of oncohematology, bone marrow transplantation, and cellular therapy describing recommendations on the Clinical Centers preparation, training of teams that will use CAR-T cells, and leading clinical questions as to their use and the management of potential complications.
The advent of the chimeric antigen receptor T-cell (CAR-T) has revolutionized how we treat hematological neoplasms. Patients with relapsed or refractory diseases, formerly with limited therapeutic options and poor prognosis, began having an effective alternative, with impressive and long-lasting response rates and, in some cases, an improvement in overall survival.1–3 However, this new therapeutic modality is associated with unique toxicities, many unknown to hematologist physicians and potentially severe or even fatal.4 The early recognition and adequate treatment of the potential complications related to the use of CAR-T cells are imperative for their success.
In this manner, considering imminent approval of some commercial CAR-T products by the Agência Nacional de Vigilância Sanitária (ANVISA), the Associação Brasileira de Hematologia, Hemoterapia e Terapia Celular (ABHH) invited a panel of specialists in oncohematology diseases, cellular therapy, and bone marrow transplantation to elaborate recommendations on this innovative therapeutic modality to Clinical Centers which will make use of it.
In this article, we describe practical recommendations for preparing Clinical Centers willing to perform CAR-T cell treatment, for the patient care before, during, and following the administration of the cellular product, and, mainly, for the recognition and management of CAR-T related toxicities. Issues related to cell-processing, regulatory and treatment indication are covered in other specific chapters of this Consensus.
Preparation of the center to perform the clinical use of CAR-T cellsThe administration of CAR-T cells should be performed at a clinical care unit with structure and expertise in cytotoxic and immunosuppressive drug use and infusion of cryopreserved or fresh cellular products.5 A trained team to promptly recognize and treat any acute or late complication due to CAR-T cells use must be available during patient hospitalization, as well as for outpatient evaluations following the discharge from the hospital.
Periodic meetings with the team composed of hematological oncologists, specialists in bone marrow transplant, and cellular therapy are recommended for the evaluation and discussion on the indication for patient inclusion in the treatment.6,7 The Center willing to perform the treatment with CAR-T cells must initially be prepared for a high demand for the service and respond with urgency, given the frequent gravity of the patient condition and absence of other alternative therapies.7 The development of a structure which facilitates and organizes the referral of outpatients with a rapid response and the establishment of well-defined criteria for eligibility is the first step before initiating and divulging the treatment. Subsequent chapters of this Consensus may assist in these definitions, which will be fundamental for offering this treatment modality to those indicated.
In Brazil, there are yet no specific regulations for Centers that will make clinical use of CAR-T. However, the regulatory entities may require the participating Centers to be included and trained in risk evaluation and mitigation strategies (REMS) programs similar to those required by the Food and Drug Administration (FDA).8,9
RecommendationsSo that a Center may be apt to perform the clinical use of CAR-T cells, the following minimum requirements are recommended:
- •
Team of hematologist physicians trained to recognize and treat CAR-T complications available 24 h / 7 days;
- •
The adequate proportion of at least one nurse for four patients in the unit where the treatment will be administered, guaranteeing the continuous monitoring of a patient;
- •
Available and nearby Intensive Care Unit (ICU), with collaboration well established among the sectors and intensivist professionals familiarized with this treatment modality and its toxicities;
- •
Team of neurologist physicians trained to recognize and treat neurological complications, such as Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS);
- •
Emergency unit with a trained team to recognize and treat CAR-T complications available following hospital discharge;
- •
Transfusion service;
- •
Immediate availability of tocilizumab (at least two doses before each treatment) at the unit where the treatment will be administered, and;
- •
All the members of the teams described above should be trained to recognize and treat all potential complications stemming from CAR-T cell therapy. Periodic meetings among groups and the elaboration of a standard operating procedures manual (SOPM) are further recommended.
These prerequisites are all (except the last) requirements for the accreditation and qualification of a bone marrow transplant (BMT) Center, either autologous or allogeneic, in Brazil.10 In this manner, this Consensus recommends that CAR-T cell treatment be performed at Centers having allogeneic or autologous BMT service already established, as in the majority of Centers in Europe and the USA.11 BMT and/or cellular therapy accreditation is also stimulated.
Patient selection and pre-treatment evaluationThe selection of eligible patients is primordial to the success and safety of the CAR-T cell treatment.12–14 Initially, it is imperative to guarantee that the patient may benefit from the treatment, selecting only carriers of pathologies for which the specific type of CAR-T cells available has already had its efficacy proven in previous clinical studies. Moreover, to mitigate therapy-associated risks, selection of patients with good performance status, without organic dysfunctions or comorbidities which potentially compromise the capacity to tolerate toxicities related to the treatment is mandartory.13 Most of the clinical studies on CAR-T cells exclude patients with organic dysfunction or poor performance status.12 Although real-life studies have recently demonstrated the safety of using CAR-T cells in older populations, with comorbidities and worse performance status,15 this conduct is not initially recommended before the Brazilian Centers gain more experience, and there has been a more extended follow-up in these studies to evaluate the risk/benefit ratio.12,13
The initial evaluation should include cardiac, renal, hepatic, and pulmonary function tests and basal neurological examination, similar to the autologous or allogeneic BMT. Furthermore, an active and non-controlled infection may be aggravated by the lymphodepletion caused by chemotherapy, B cell aplasia induced by CAR-T (in anti-CD19 products), or even increase acute toxicities due to elevated basal levels of interleukins. Basal serological evaluation is also recommended, as for autologous or allogeneic BMT.12
Active autoimmune diseases can increase the toxicity of CAR-T cells treatment. Moreover, immunosuppressives can interfere in T cells collection, CAR-T cell function, and cellular manufacturing process.13
There is a variable and considerable time lapse between patient inclusion and the hospitalization to receive treatment (time necessary for cellular product manufacturing). It is recommended that this same evaluation be repeated before initiating the lymphodepletion regime.
Recommendations- •
CAR-T cells indications should be approved on the commercial product label (type of disease, age of the patient, and treatment line). In clinical trials, must respect the inclusion and exclusion criteria;
- •
Patients with a good performance status (ECOG 0-2) and without severe organic dysfunctions should be eligible for the treatment with CAR-T cells;
- •
Treatment with CAR-T cells is not recommended for patients with active autoimmune disease resulting in end-organ injury or requiring systemic immunosuppression;
- •
Patients with non-controlled active infection should be adequately treated, and the CAR-T cell therapy postponed until its resolution or control;
- •
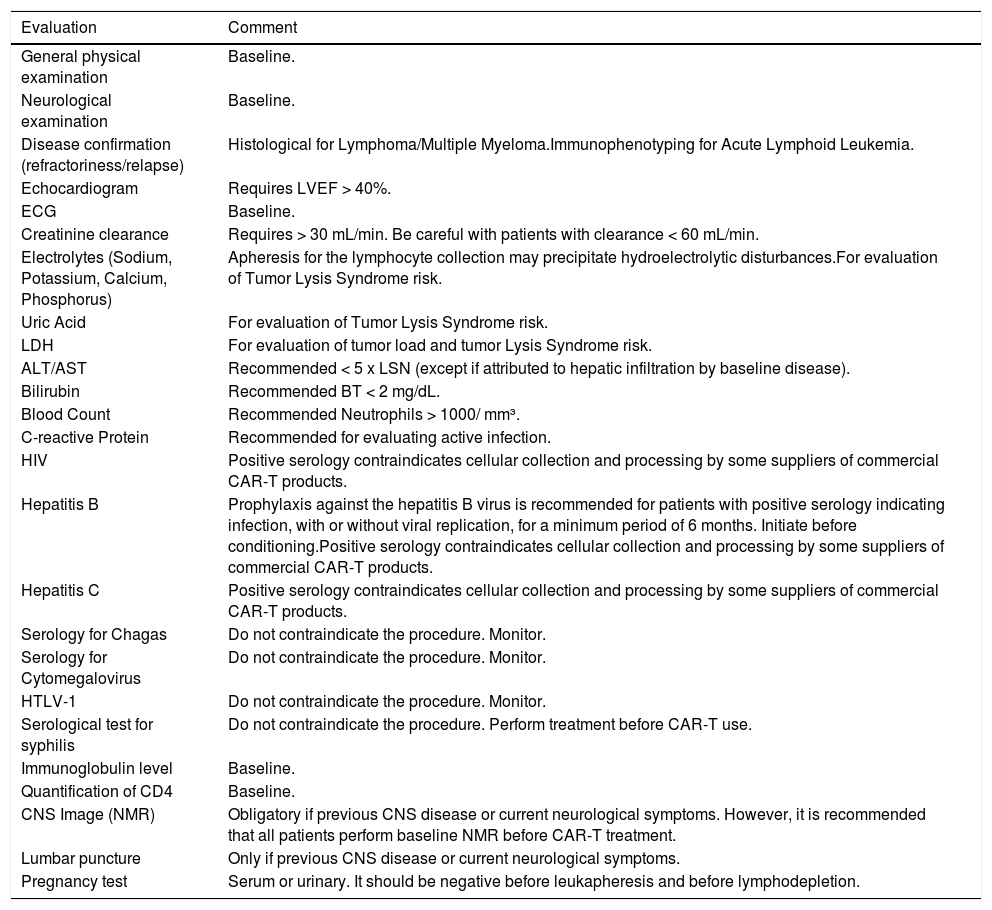
Tests recommended for initial evaluation (eligibility), and those before the initiation of lymphodepletion are described in Table 1, and;
Table 1.Recommended minimum evaluation for determination of eligibility to CAR-T cells clinical use.
LVEF: Left ventricular ejection fraction; ECG: Electrocardiogram; LDH: Lactate dehydrogenase; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; HIV: Human immunodeficiency virus; HTLV-1: Human T-lymphotropic virus type 1; CNS: Central nervous system; NMR: Nuclear Magnetic Resonance.
Adapted from: Yakoub-Agha I, et al. Haematologica, 202012.
- •
We recommend that an informed consent form (ICF) be obtained from the patient and/or its guardian before any procedure, similar to what already occurs for BMT.
Conditioning with lymphodepletion chemotherapeutic schemes before CAR-T cells infusion increases the efficacy of the treatment.16 Multiple mechanisms, such as immune suppressor cells elimination (regulatory T-cells, for example), reduction of T, B, and NK cells, as well as an increase in homeostatic cytokines (IL-7 and IL-15), and an increase in tumor cell co-stimulatory molecules promote a favorable environment for the expansion and survival of in vivo CAR-T cells.17
Fludarabine and cyclophosphamide combination was superior to cyclophosphamide alone or cyclophosphamide plus etoposide18 and is currently more widely used and recommended. However, various other schemes containing bendamustine, pentostatin, total body irradiation (TBI), and polychemotherapy, although possible, are seldom used.12–14
Recommendations- •
Initiate conditioning only following the confirmation of the availability of the cellular product (CAR-T cells) for the patient, considering the product estimated date of arrival at the Center;
- •
Conditioning should be performed in all patients, independent of previous leukocyte or lymphocyte counts;
- •
Fludarabine and cyclophosphamide regimen is recommended for conditioning; and
- •
CAR-T cells may cause rapid destruction of tumor cells. Tumor lysis prophylaxis is recommended, as per risk and institutional protocol, considering the type of disease, tumor load, and comorbidities.
The infusion of cellular products is generally safe and well-tolerated, although severe adverse reactions may occur and should be monitored. For such, the patient must be hospitalized and monitored for vital signs, oxygen saturation, and urinary output.12,14 Emergency materials, aspiration, and oxygen should be available at the patient's bedside.
Characteristic reactions, such as nausea, vomiting, abdominal pain, fever, and tremors, are the most frequent. Rarely, respiratory depression, cardiac arrhythmias, and neurological symptoms may be observed.19 The general principles for managing these reactions include reducing the velocity or halting of the infusion, standard emergency conduct, and the confirmation of the receptor/product.
Main acute complications generally occur within 28 days of the CAR-T cells infusion and frequently after hospital discharge.12 Thus, the patient should remain near the Center responsible for the CAR-T cells clinical use, having prompt access to emergency support service during this period.
Recommendations- •
CAR-T cells should be infused with the patient hospitalized, with a minimum intrahospital observation for 14 days. This recommendation stems from the Centers gaining experience with this treatment modality;
- •
There should be a two-person (double-check) verification of the product data and confirmation of the receptor identification before installing the cells;
- •
Must use connections or transfusion equipment without a leukocyte filter;
- •
Paracetamol and an antihistamine (diphenhydramine) should be used as pre-medication, 30 to 60 minutes before cell infusion;
- •
Corticosteroids should not be used previously to or immediately after CAR-T cells infusion (except in cases of anaphylactic reaction);
- •
No medication should be infused in the same venous line concomitantly with CAR-T cells;
- •
A rapid infusion is recommended, in approximately 30 min;
- •
The patient should be monitored during the whole procedure and in the subsequent hours;
- •
The preparation and supply of materials enlightening patients on the treatment and possible complications is recommended. A pocket card with orientations on the alert signs/symptoms and contact information of the Center responsible for CAR-T clinical use should be in the patient's possession following the hospital discharge,8,9 and;
- •
The patient should remain close to the Center responsible for CAR-T clinical use (up to two hours away is recommended) for at least four weeks following the infusion.
The two most frequent CAR-T cell-related complications are cytokine release syndrome (CRS) and ICANS. These toxicities can be severe or even fatal, and early recognition is crucial for their management. CRS and ICANS occur days to weeks following CAR-T cells infusion (depending on the product infused), when most of the patients also have post-chemotherapy pancytopenia, and associated infections may complicate the diagnosis and treatment of these syndromes.
Projects with CAR-T cells are under development in Brazilian research centers, and different products are being approved for commercial use in Brazil. The clinical presentation of CRS and ICANS is similar for the various products; however, the initiation time, incidence, and severity can significantly vary. Products’ particularities, such as the type of cells used in the production (mononuclear cells vs. T cells), differences in the fabrication process, co-stimulatory molecule (CD28 vs. 4-1BB), CAR-T cells dose, and the type and intensity of the lymphodepletion chemotherapy, as well as patient-specific factors, such as age, diagnosis, and tumor load, may influence in these differences. Clinical studies with anti-CD19 CAR-T cells containing CD28 as the co-stimulatory molecule (e.g., axicabtagene ciloleucel) suggest greater risk and severity of CRS and ICANS when compared to products containing 4-1BB.
Considering these particularities, this Consensus presents recommendations for CRS and ICANS management, primarily in adults. Management of CRS and ICANS is based on the American Society for Transplantation and Cellular Therapy (ASTCT) classification system.20 For commercial products, additional recommendations present in the package insert and in the REMS should also be taken into consideration.8,9
Cytokine Release Syndrome (CRS)CRS presents as a systemic inflammatory response syndrome with fever (not attributed to any other cause). It is fundamental to evaluate, treat and prevent systemic infection, as most patients present with febrile neutropenia and various CRS manifestations mimic sepsis. Moreover, infectious conditions and systemic inflammation can exacerbate the CRS. In this manner, both possibilities should be considered and treated in a patient with fever within the first weeks following CAR-T cell therapy.
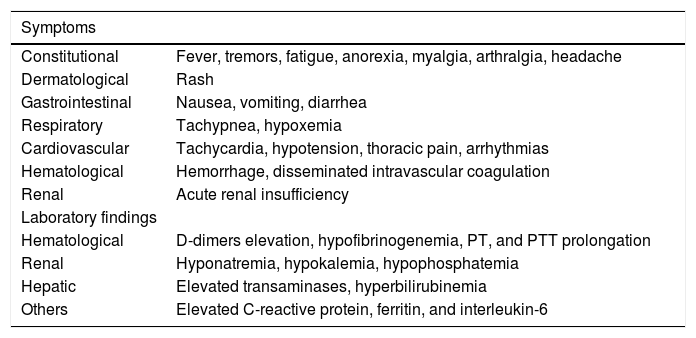
Table 2 presents the main symptoms and laboratory findings of CRS. Following CRS diagnosis, the persistence of fever is not necessary for CRS classification.
Symptoms and laboratory findings in CRS.
It is recommended to evaluate vital signs every 4 h following CAR-T cell infusion, and daily blood count, renal function, electrolytes, hepatic function, lactate dehydrogenase, coagulation tests (including fibrinogen and d-dimers), uric acid, c-reactive protein (CRP), and ferritin.
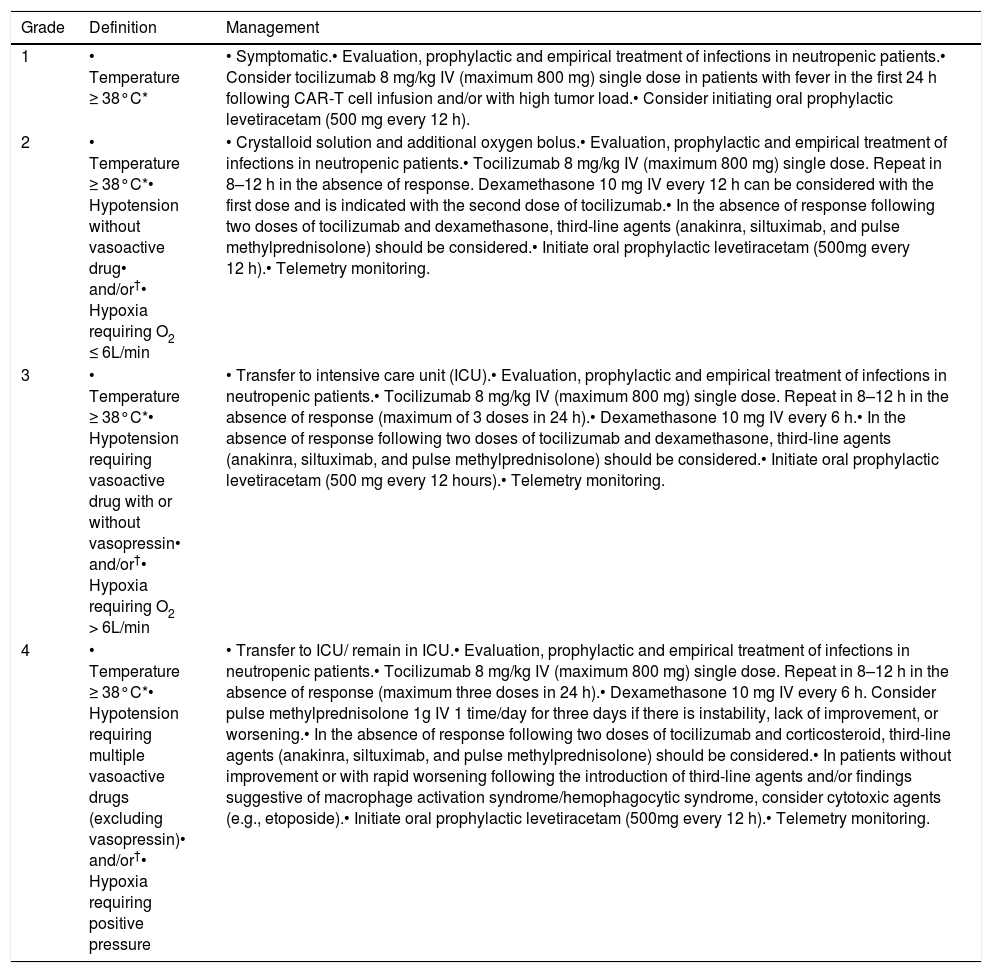
Table 3 presents recommendations for the management based on the ASTCT classification system. CRS treatment is based on the use of tocilizumab (anti-interleukin-6 receptor (IL-6) antibody) or other anti-IL-6 therapy available/approved in the country. The hospitals/centers should maintain at least two anti-IL-6 doses available for administration in up to two hours.
CRS classification and management.
| Grade | Definition | Management |
|---|---|---|
| 1 | • Temperature ≥ 38°C* | • Symptomatic.• Evaluation, prophylactic and empirical treatment of infections in neutropenic patients.• Consider tocilizumab 8 mg/kg IV (maximum 800 mg) single dose in patients with fever in the first 24 h following CAR-T cell infusion and/or with high tumor load.• Consider initiating oral prophylactic levetiracetam (500 mg every 12 h). |
| 2 | • Temperature ≥ 38°C*• Hypotension without vasoactive drug• and/or†• Hypoxia requiring O2 ≤ 6L/min | • Crystalloid solution and additional oxygen bolus.• Evaluation, prophylactic and empirical treatment of infections in neutropenic patients.• Tocilizumab 8 mg/kg IV (maximum 800 mg) single dose. Repeat in 8–12 h in the absence of response. Dexamethasone 10 mg IV every 12 h can be considered with the first dose and is indicated with the second dose of tocilizumab.• In the absence of response following two doses of tocilizumab and dexamethasone, third-line agents (anakinra, siltuximab, and pulse methylprednisolone) should be considered.• Initiate oral prophylactic levetiracetam (500mg every 12 h).• Telemetry monitoring. |
| 3 | • Temperature ≥ 38°C*• Hypotension requiring vasoactive drug with or without vasopressin• and/or†• Hypoxia requiring O2 > 6L/min | • Transfer to intensive care unit (ICU).• Evaluation, prophylactic and empirical treatment of infections in neutropenic patients.• Tocilizumab 8 mg/kg IV (maximum 800 mg) single dose. Repeat in 8–12 h in the absence of response (maximum of 3 doses in 24 h).• Dexamethasone 10 mg IV every 6 h.• In the absence of response following two doses of tocilizumab and dexamethasone, third-line agents (anakinra, siltuximab, and pulse methylprednisolone) should be considered.• Initiate oral prophylactic levetiracetam (500 mg every 12 hours).• Telemetry monitoring. |
| 4 | • Temperature ≥ 38°C*• Hypotension requiring multiple vasoactive drugs (excluding vasopressin)• and/or†• Hypoxia requiring positive pressure | • Transfer to ICU/ remain in ICU.• Evaluation, prophylactic and empirical treatment of infections in neutropenic patients.• Tocilizumab 8 mg/kg IV (maximum 800 mg) single dose. Repeat in 8–12 h in the absence of response (maximum three doses in 24 h).• Dexamethasone 10 mg IV every 6 h. Consider pulse methylprednisolone 1g IV 1 time/day for three days if there is instability, lack of improvement, or worsening.• In the absence of response following two doses of tocilizumab and corticosteroid, third-line agents (anakinra, siltuximab, and pulse methylprednisolone) should be considered.• In patients without improvement or with rapid worsening following the introduction of third-line agents and/or findings suggestive of macrophage activation syndrome/hemophagocytic syndrome, consider cytotoxic agents (e.g., etoposide).• Initiate oral prophylactic levetiracetam (500mg every 12 h).• Telemetry monitoring. |
Modified from Lee DW, et al. Biol Blood Marrow Transplant. 2019.20
In the Brazilian Centers’ initial experience with CAR-T cells, it will be important that intensivist physicians be aware of the CAR-T cell infusions and follow CRS management, even in low-grade cases, so that there will be greater continuity in the treatment of these patients. The same should occur with neurologists and infectious disease specialists physicians.
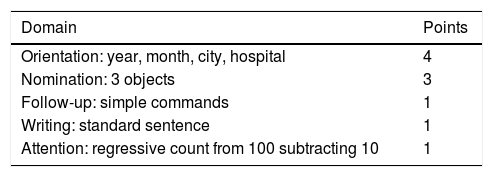
Cell-Associated Neurotoxicity Syndrome (ICANS)Neurotoxicity mainly occurs in the first 1–2 weeks following CAR-T cell infusion and rarely later on. ICANS evaluation involves using the Immune Effector Cell Encephalopathy (ICE) scale, with a 0–10 point system (Table 4). Patients should be submitted to a basal neurological evaluation, including the ICE scale, with daily revaluation from infusion until the 21st day following the administration of the CAR-T cells. Antiepileptic prophylaxis with agents, such as levetiracetam, is not routinely recommended, except for patients with a history of convulsions or central nervous system disease.12
ICE scale.
| Domain | Points |
|---|---|
| Orientation: year, month, city, hospital | 4 |
| Nomination: 3 objects | 3 |
| Follow-up: simple commands | 1 |
| Writing: standard sentence | 1 |
| Attention: regressive count from 100 subtracting 10 | 1 |
Modified from Lee DW, et al. Biol Blood Marrow Transplant. 2019.20
Table 5 presents the management recommendations based on the ASTCT classification system. Tocilizumab does not cross the hematoencephalic barrier and is associated with an increase in IL-6 serum levels and potentially greater risk and severity of ICANS due to the rise in IL-6 levels in the central nervous system. For this reason, tocilizumab should not be used for the treatment of ICANS in the absence of CRS.
Classification and management of ICANS.
| Grade | ICE Scale | Consciousness level† | Epileptic seizure | Motor findings‡ | Intracranial hypertension/ cerebral edema§ | Management |
|---|---|---|---|---|---|---|
| 1 | 7–9 | Rouses spontaneously | NA | NA | NA |
|
| 2 | 3–6 | Rouses with auditory stimulus | NA | NA | NA |
|
| 3 | 0–2* | Rouses only with tactile stimulus | Any epileptic seizure, focal or generalized with rapid resolution or non-convulsive seizure on EEG which respond to intervention | NA | Focal/ local edema in neuroimage |
|
| 4 | 0* | Unconscious or requiring vigorous and repetitive tactile stimulation to wake up. Stupor or coma. | Prolonged epileptic seizure (> 5 min) or state of epileptic malaise. | Focal deep muscular weakness | Diffuse cerebral edema in neuroimage; decerebration or decortication; paralysis of VI cranial pair; papilledema; Cushing triad |
|
Modified from Lee DW, et al. Biol Blood Marrow Transplant. 201920.
Patients with ICE scale 0 can be classified as ICANS grade 3 if conscious and with global aphasia and ICANS grade 4 if unconscious and incapable of performing the ICE scale.
Infections related to CAR-T cell therapy are prevalent events. Febrile neutropenia grade ≥ 3 occurs in approximately 15 to 35% of cases in the first four to eight weeks following infusion.12,21–24 During the first weeks, bacteremia, fungal infections, upper respiratory infections, herpes zoster, and viral reactivations, such as cytomegalovirus, Epstein Barr virus, and human herpesvirus-6 may appear25; and after 30 days, viral respiratory infections, cytomegalovirus, and pneumonia predominate.12,22,23,26 Invasive fungal infections are rare and mainly observed in ALL patients who have previously had allogeneic BMT.12
Described risk factors for CAR-T-related infection are more than four previous chemotherapy schemes, infusion of high CAR-T dose, CRS grade ≥ 3, use of systemic corticosteroid, and serum IgG < 400 mg/dL.23,26–29
Recommendations- •
Routine antibacterial prophylaxis is not recommended;
- •
Use viral prophylaxis with acyclovir 400 mg every 12 h (Pediatric dose: 60–90 mg/kg/day - maximum of 800mg, divided into 2 or 3 doses. Children and Adolescents ≥40 kg: same dose as for adults) or valaciclovir 500 mg every 12 h (Pediatric dose for patients < 40 kg: 250 mg. Children and Adolescents ≥ 40 kg: same dose as for adults)12,25; for 6–12 months following CAR-T infusion, preferentially suspending only after CD4 > 200/mm³;
- •
Use prophylaxis for pneumocystis with sulfamethoxazole/trimethoprim 800/160 mg/day (Pediatric dose: 5–10 mg/kg/day of trimethoprim), 3 times per week12,25 for 6–12 months following CAR-T infusion, preferentially suspending only after CD4 > 200 /mm3;
- •
Antifungal prophylaxis with fluconazole is recommended for patients using corticosteroid ≥ 0.5 mg/kg/day and during the period of neutropenia. Add filamentous fungi prophylaxis in the presence of two or more of the following risk factors: ≥ 4 previous chemotherapy treatments, neutropenia (< 500/mm³) before CAR-T infusion, CAR-T dose over 2 × 107/kg, previous invasive fungal infection, and use of tocilizumab and/or corticosteroids.29 The choice of the best antifungal agent is at the discretion of each institution, local epidemiology, and previous patient's history;
- •
Infectious vigilance and adequate antimicrobial prophylaxis are recommended for patients who had received immunosuppressive treatment for CRS and ICANS and patients with prolonged neutropenia using corticosteroids12,25;
- •
Prophylaxis against hepatitis B virus is recommended for patients with positive serology indicating previous infection, with or without viral replication, initiating before the conditioning and maintained for a minimum of 6 months12,30;
- •
Start vaccinations six months after CAR-T cell infusion,30 and;
- •
Influenza30 and SARS-CoV-231 vaccinations are recommended, completing the scheme two weeks before CAR-T cells infusion.
Cytopenias are very frequent following anti-CD19 CAR-T cells treatment and are also associated with previous treatments and lymphodepletion before CAR-T infusion. Grade ≥ 3 neutropenia occurs in up to 78% of patients; grade ≥3 anemia in up to 43%, and grade ≥3 thrombocytopenia in up to 38% of patients.32 The risk factors for CAR-T-related cytopenias are grade ≥3 CRS/ICANS, pre-existing cytopenias, and possibly the type of product used (a previous study showed fewer cytopenias following Tisa-Cel).33 These cytopenias can persist three months after CAR-T infusion in approximately 32% of cases24 and may last even longer.
Levels of CD8+ and CD56+ lymphocytes persist relatively stable following anti-CD19 CAR-T infusion. In contrast, CD4+ lymphocytes remain low, even one year after CAR-T infusion, with a median of approximately 150–250 cells/mm³,21,27,34,35 and CD19+ lymphocytes recovery inversely correlates with the persistence of anti-CD19 CAR-T cells.36,37
Recommendations- •
Cytopenias lasting over 28 days: proceed with bone marrow aspirate and biopsy to rule out viral infection, myelodysplasia, and disease relapse25;
- •
G-CSF can be used to reduce the period of neutropenia (if grade ≥ 3).12 However, some groups advocate G-CSF suspension during the CRS period due to theoretical risk of worsening. GM-CSF is not recommended25;
- •
Platelets and RBCs transfusion, as per institutional protocol;
- •
Platelet transfusion refractoriness may occur more frequently during CRS;
- •
Patients already in outpatient management, with active infections or febrile neutropenia, should be hospitalized and treated according to institutional protocols,25 and;
- •
Evaluate baseline CD4 and serum IgG levels and at 3, 6, and 12 months following CAR-T infusion .
Hypogammaglobulinemia is due to the “on-target/off-tumor” effect of anti-CD19 and anti-BCMA CAR-T cells. Almost all patients who respond to anti-CD19 CAR-T therapy develop B-cell aplasia following the infusion,25 which can persist for months to years.12 Up to 60% of patients present with persistent hypogammaglobulinemia 90 days after the treatment,21,22,37 and serum IgG levels appear to reach their nadir approximately six months after CAR-T. In some diseases, 74% of patients may have previous low baseline IgG levels, even before CAR-T therapy. Many of them due to previous anti-CD20 monoclonal antibodies.22,38
Despite the hypogammaglobulinemia, late infections following CAR-T are predominantly caused by respiratory viruses and do not lead to hospitalization. Many patients maintain protective levels of vaccine-induced antibodies and against viruses, despite CD19 and serum IgG levels,21,37,39 which appears to be associated with plasmocytes persistence following CD19+ lymphocytes depletion. Children with fewer plasmocytes can be more susceptible to infections than adults,30 and these levels might be lower following anti-BCMA CAR-T.40 Pathogens-specific IgG levels were similar for most pathogens in post-CAR-T cell therapy, with and without intravenous immunoglobulin replacement, except for S. pneumoniae.40 Given these data, costs, and risks associated with intravenous immunoglobulin infusion, we do not recommend its use based solely on serum IgG levels. We suggest intravenous immunoglobulin replacement guided by clinical conditions (ex. patients with severe or recurrent infections and serum IgG < 400 mg/dL). For patients with serum IgG > 400 mg/dL and severe and/or recurrent infections, consider dosage of IgG subclasses and pathogen-specific IgG titers.30,37
Recommendations- •
We recommend monitoring B-cells quantification and serum immunoglobulin levels monthly, for at least three months, following CAR-T cells infusion. After six months, monitoring if clinically indicated25,41;
- •
Adults with recurrent or severe infections, and children with serum immunoglobulin level less than 400 mg/dl, should be considered for monthly intravenous immunoglobulin infusion25,37;
- •
The recommended dose is the one that will maintain serum IgG level over 400 mg/dL or normal for the age. Start 400–600mg/kg every 3–4 weeks, and increase dose or frequency of infusions by clinical criteria.29 For children, start 0.5g/kg/monthly.
CAR-T cells therapy is certainly an advance in hematology. It is an effective treatment with a manageable safety profile for some patients with refractory or relapsed oncohematologic diseases. However, this new therapeutic modality also brings further complications and adverse events. The hematologist is not familiar with the CRS and ICANS, which should be expected and promptly approached. Other potential complications, also frequent in regular oncohematologic treatments, such as cytopenias, infections, and hypogammaglobulinemia, present particularities that should be understood. Therefore, it is essential that the multidisciplinary teams that will care for these patients be previously trained and capacitated to recognize and treat these complications; and that the Centers that will perform this type of treatment have an adequate structure. The selection of the patient who will benefit from and tolerate the therapy is another fundamental pillar for the clinical use of CAR-T cells.