Distinction between mature B-cell neoplasms can be difficult due to overlapping of immunologic features and clinical manifestations. This study investigated whether quantifying mean fluorescence intensity of four monoclonal antibodies in a flow cytometry panel is useful for the differential diagnosis and characterization of these disorders.
MethodsThe expressions of CD52, CD200, CD123 and CD43 were analyzed in samples from 124 patients with mature B-cell neoplasms. The quantitative estimation of these antigens was assessed by mean fluorescence intensity.
ResultsThe cases included were 78 chronic lymphocytic leukemias, three atypical chronic lymphocytic leukemias, six marginal zone lymphomas, 11 splenic marginal zone lymphomas, nine lymphoplasmacytic lymphomas, six mantle cell lymphomas, two hairy cell leukemias, two hairy cell leukemias variant, five follicular lymphomas, one Burkitt lymphoma and one diffuse large B-cell lymphoma. The mean fluorescence intensity of CD200 was higher in atypical chronic lymphocytic leukemia, chronic lymphocytic leukemia and hairy cell leukemia cases. CD123 showed higher mean fluorescence intensities in hairy cell leukemia cells. Chronic lymphocytic leukemia, atypical chronic lymphocytic leukemia and mantle cell lymphoma had higher expression of CD43 and all follicular lymphoma cases had very low mean fluorescence intensity values. CD52 expression was consistently positive among all cases.
ConclusionQuantitative evaluation of these markers can be a useful additional tool to better identify some types of mature B-cell neoplasms.
Mature B-cell neoplasms (MBCN) account for around 80% of all lymphoid neoplasms and comprise a broad spectrum of disorders with different morphologies, clinical aspects, genetics and immunophenotypes. However, they have a more mature lymphoid progenitor in common compared to immature neoplasms. According to the World Health Organization (WHO), immunophenotypic similarities of these cells at a certain stage of maturation in conjunction with morphological, genetic and clinical findings allow these diseases to be classified and diagnosed.1
Immunophenotyping by flow cytometry is a fast and cost-effective technique widely used for the diagnosis and follow up of hematological disorders. Multiparametric flow cytometry (MFC) has become more complex in the diagnosis of MBCN due to the availability of several markers, in addition to the high number of entities. Usually, the analysis is performed separately for each sample aliquot labeled with four to eight monoclonal antibodies (MoAbs); an expert interprets the immunophenotypic profile of neoplastic B-cells and specifies a diagnosis. Preferred markers are those able to differentiate B-cells from other cells, define the maturation stage and identify phenotypic aberrations. Among these are the pan B-cell markers (CD19 and CD20) and those used for the differential diagnosis (CD10, CD5, CD103, CD43, CD23, CD49d, CD81, CD200, CXCR5, immunoglobulin (Ig)M and Kappa and Lambda chains). A differential diagnosis can be difficult, due to overlapping immunophenotypic features and similar clinical manifestations, such as cases of differentiation between lymphoplasmacytic lymphoma (LPL) and marginal zone lymphoma (MZL), or between diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma. For the final diagnosis, it is important to integrate results of MFC with morphological, clinical and cytogenetic analysis.1,2
Some markers are known for their expression in MBCN, such as the positivity of CD52,3 and negativity of CD43 in MZL,4 the usefulness of CD200 in the differential diagnosis between chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL),5 and the role of CD123 in the diagnosis of hairy cell leukemia (HCL).6 However, the quantitative analysis of these markers in MCBN is seldom discussed, and its expression may be important not only for the differential diagnosis, but also for prognosis and potential therapeutic factors, given the existence of target drugs against some of these markers.
The aim of this study was to evaluate quantitatively the expression of CD200, CD123, CD43 and CD52 in MBCN.
MethodsAll MCBN cases diagnosed in a reference laboratory in the south of Brazil from October 2014 to June 2015 were consecutively included in the study. Specimens sent for reassessment were not included. The study was approved by the local Ethics Committee; written informed consent was deemed unnecessary after the signature of a commitment term for the use of biological material and associated information by researchers.
The panel with the four MoAbs under study was distributed using the following fluorescences: CD123 FITC (clone 7G3), CD52 PE (clone 4C8), CD43 APC (clone 1G10) and CD200 PerCP-Cy5.5 (clone MRC OX-104). All MoAbs were purchased from BD Biosciences (San Diego, USA). About 1,000,000 cells from whole blood or bone marrow samples anticoagulated with EDTA were incubated for 20min in the dark at room temperature with each MoAb. Red blood cells were lysed by incubation with Excellyse I (EXBIO, Praha, CZ), followed by incubation with distilled water. Samples were washed and resuspended in phosphate buffered saline (PBS). All reagents were used according to the manufacturer's instructions. All samples were processed within 48h of collection.7
Immediately after preparation, samples were analyzed on a FACSCalibur flow cytometer using CellQuest™ Pro software (BD Biosciences, San Diego, CA, USA). About 100,000 events per sample were obtained. Infinicity™ Flow Cytometry version 1.7 software (Cytognos, SL, ES) was used for data analysis. For the gating strategy, debris were removed, based on forward-scatter (FSC) and side-scatter (SSC) distribution. Neoplastic cells were initially identified by positivity of the CD19 and CD20 and then in accordance with the expression of other panel markers for MBCN. The mean fluorescence intensities (MFIs) of neoplastic cells were recorded in arbitrary units from 0 to 104.
Reproducibility of the fluorescence intensities was preserved by calibration and daily quality control procedures. Calibrite beads (BD Biosciences, Sao Diego, USA) were used in order to ensure standardization. All diagnosis were established in accordance with the WHO criteria.1
Medians and interquartile ranges for each MFI of MoAb were calculated. The Mann–Whitney test was used to calculate the statistical significance of differences between groups. Burkitt lymphoma and DLBCL were not included in the statistical analysis because there was only one case of each disease. Differences were considered significant when the p-value <0.05. All statistical analyses were performed using the Statistical Package for the Social Sciences v. 18.0 software (SPSS, Chicago, USA).
ResultsImmunophenotypic analysis was performed in 124 samples – 67 of peripheral blood (54%) and 57 of bone marrow (46%) – from patients diagnosed with MBCN. Patient mean age was 69.3±12.1 years old and 51.6% were men. The distribution of differential diagnoses of MBCN is described in Table 1. The most common diagnosis, as expected, was CLL due to its high prevalence, and the rarest were Burkitt lymphoma and DLBCL. The rate of neoplastic B-cells among total cells per sample ranged from 3.7% to 97.1%, with a mean of 52.3±25.4%. Median MFIs for each MoAb of the different groups are shown in Table 2.
Distribution of mature B-cell neoplasms.
| Diagnosis | Number of cases | (%) |
|---|---|---|
| Atypical chronic lymphocytic leukemia | 3 | 2.4 |
| Burkitt lymphoma | 1 | 0.8 |
| Chronic lymphocytic leukemia | 78 | 62.9 |
| Diffuse large B-cell lymphoma | 1 | 0.8 |
| Follicular lymphoma | 5 | 4.0 |
| Hairy cell leukemia | 2 | 1.6 |
| Hairy cell leukemia variant | 2 | 1.6 |
| Lymphoplasmacytic lymphoma | 9 | 7.3 |
| Mantle cell lymphoma | 6 | 4.8 |
| Marginal zone lymphoma | 6 | 4.8 |
| Splenic marginal zone lymphoma | 11 | 8.9 |
Median MFI values of evaluated markers per disease category.
| Diagnosis | CD200 | CD123 | CD43 | CD52 |
|---|---|---|---|---|
| Atypical chronic lymphocytic leukemia | 113.7 (70.4/122.2) | 7.5 (5.7/7.6) | 133.7 (74.5/154.3) | 765.2 (546.5/814.7) |
| Burkitt lymphoma | 4.5 (4.5/4.5) | 5.9 (5.9/5.9) | 57.0 (57.0/57.0) | 143.0 (143.0/143.0) |
| Chronic lymphocytic leukemia | 61.1 (41.4/89.2) | 8.0 (5.6/10.7) | 179.8 (112.8/278.7) | 777.9 (469.3/1101.0) |
| Diffuse large B-cell lymphoma | 3.8 (3.8/3.8) | 6.1 (6.1/6.1) | 160.9 (160.9/160.9) | 549.5 (549.5/549.5) |
| Follicular lymphoma | 2.6 (1.8/11.4) | 6.4 (4.5/10.0) | 6.8 (3.6/13.1) | 686.1 (455.9/820.2) |
| Hairy cell leukemia | 220.3 (163.1/277.5) | 83.2 (61.3/105.0) | 79.0 (44.4/113.7) | 956.4 (911.3/1001.6) |
| Hairy cell leukemia variant | 36.1 (22.1/50.1) | 22.0 (14.4/29.5) | 14.6 (11.9/17.2) | 990.3 (705.9/1274.8) |
| Lymphoplasmacytic lymphoma | 14.0 (12.30/40.2) | 9.0 (6.7/11.1) | 20.7 (6.5/25.3) | 697.1 (447.7/1150.8) |
| Mantle cell lymphoma | 3.5 (2.1/4.1) | 13.9 (8.6/30.9) | 90.1 (48.6/226.7) | 704.5 (538.7/1375.5) |
| Marginal zone lymphoma | 8.3 (4.5/13.2) | 11.7 (8.1/16.0) | 7.8 (5.0/23.6) | 1326.1 (737.5/1847.0) |
| Splenic marginal zone lymphoma | 7.6 (4.0/22.9) | 12.3 (9.4/16.1) | 7.1 (6.5/8.6) | 1297.6 (1096.9/1861.8) |
Data are shown as median (percentile 25/75).
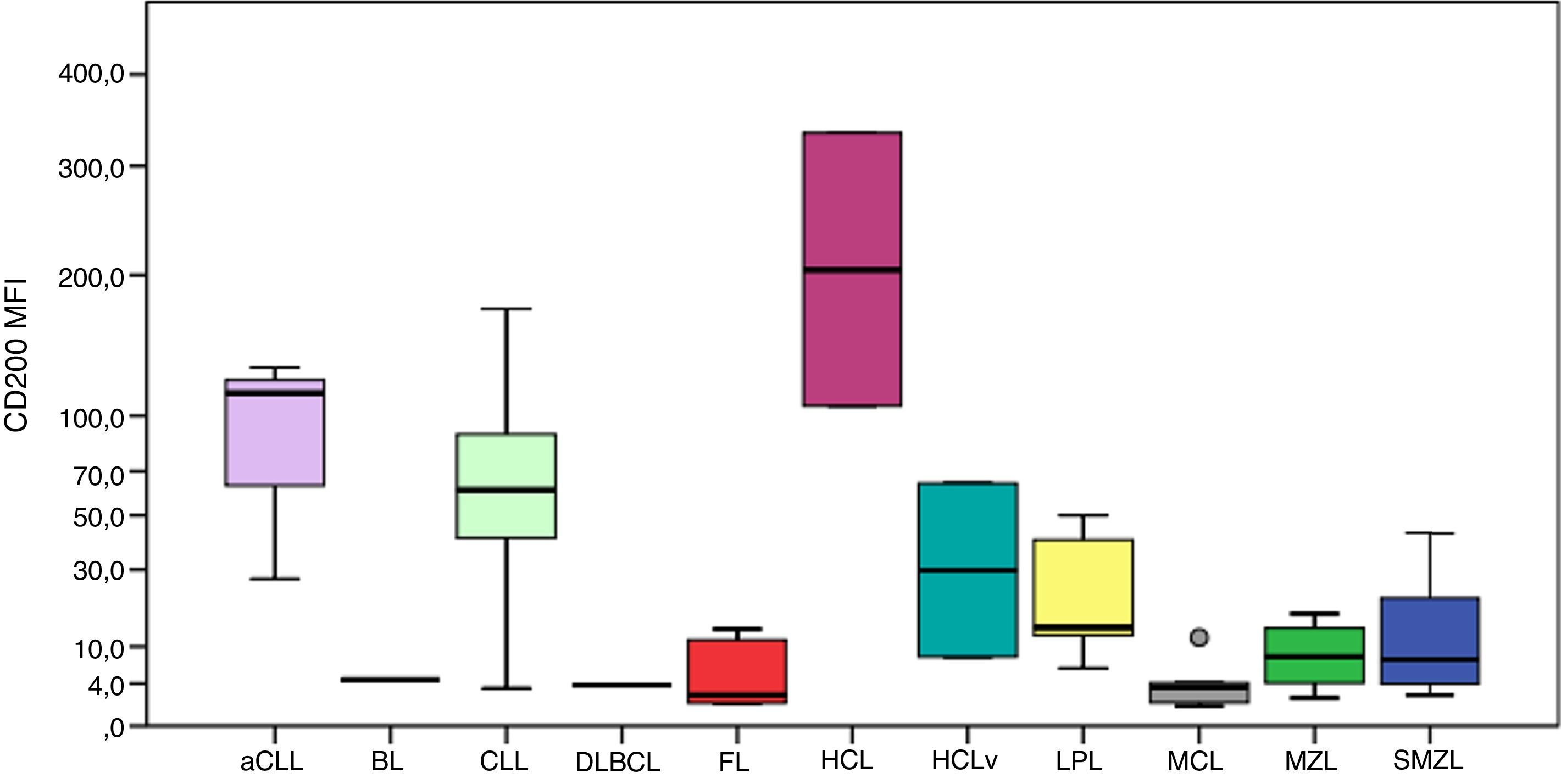
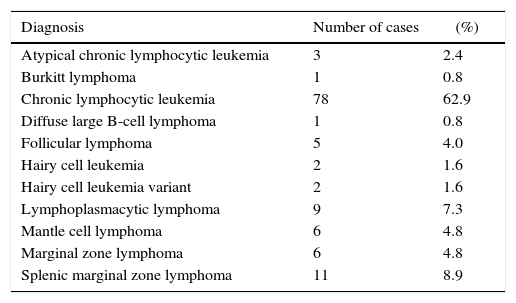
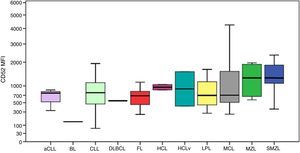
The MFI of CD200 was higher in atypical chronic lymphocytic leukemias (aCLL), CLL and HCL as can be seen in Figure 1. The MFI of CD200 was higher in aCLL compared to MCL (p-value=0.020), follicular lymphoma (p-value=0.034), MZL (p-value=0.020) and splenic marginal zone lymphomas (SMZL) (p-value=0.036). The MFI of CD200 was higher in CLL compared to LPL (p-value <0.001), MCL (p-value <0.001), follicular lymphoma (p-value=0.001), MZL (p-value <0.001) and SMZL (p-value <0.001). The MFI of CD200 was higher in LPL compared to MCL (p-value=0.003). The MFI of CD200 was higher in HCL compared to MCL (p-value=0.046), MZL (p-value=0.046) and SMZL (p-value=0.030). The MFI of CD200 of HCL was about 6-fold higher when compared to hairy cell leukemia variant (HCLv), however due to the small number of cases included it was not possible to show statistical difference in CD200 expression between these two neoplasms (p-value=0.121).
Boxplot showing MFI of CD200 in 124 cases of mature B-cell neoplasms. aCLL: atypical chronic lymphocytic leukemia; BL: Burkitt lymphoma; CLL: chronic lymphocytic leukemia; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; HCL: hairy cell leukemia; HCLv: hairy cell leukemia variant; LPL: lymphoplasmacytic lymphoma; MCL: mantle cell lymphoma; MZL: marginal zone lymphoma; SMZL: splenic marginal zone lymphoma.
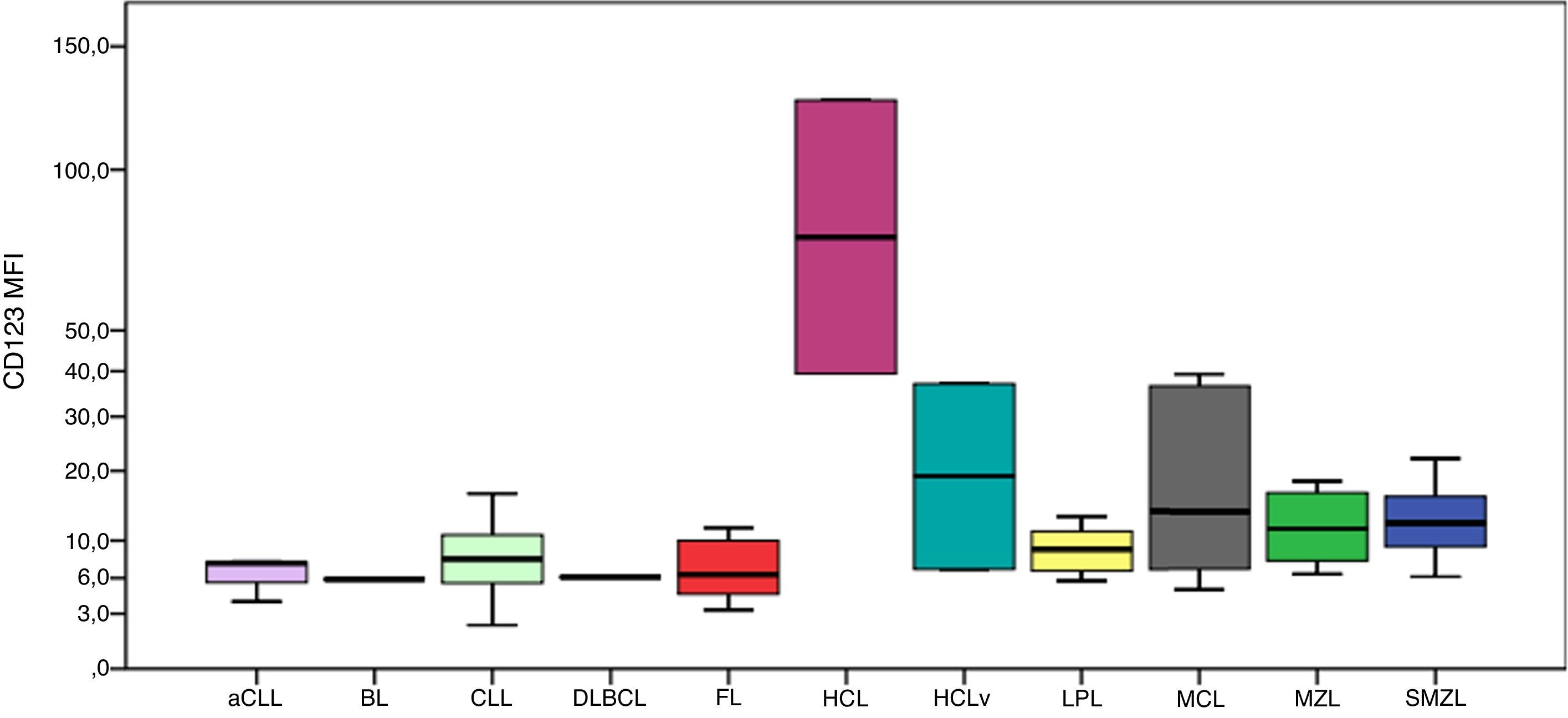
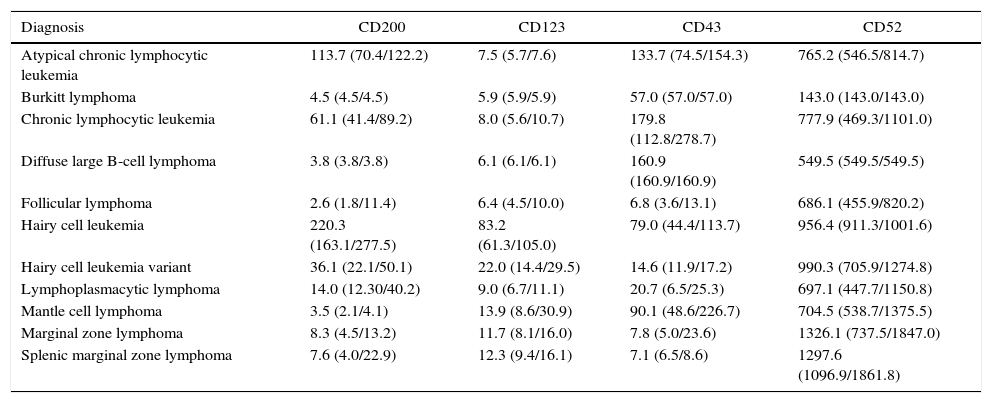
CD123 had higher MFIs in HCL cells as shown in Figure 2. The MFI of CD123 was higher in HCL compared to CLL (p-value=0.016), LPL (p-value=0.034), MCL (p-value=0.046), MZL (p-value=0.046) and SMZL (p-value=0.030). The MFI of CD123 was higher in SMZL compared to CLL (p-value=0.003), LPL (p-value=0.037) and follicular lymphoma (p-value=0.050). MFI of CD123 had a trend to be higher in HCL compared to HCLv (p-value=0.121).
Boxplot showing MFI of CD123 in 124 cases of mature B-cell neoplasms. aCLL: atypical chronic lymphocytic leukemia; BL: Burkitt lymphoma; CLL: chronic lymphocytic leukemia; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; HCL: hairy cell leukemia; HCLv: hairy cell leukemia variant; LPL: lymphoplasmacytic lymphoma; MCL: mantle cell lymphoma; MZL: marginal zone lymphoma; SMZL: splenic marginal zone lymphoma.
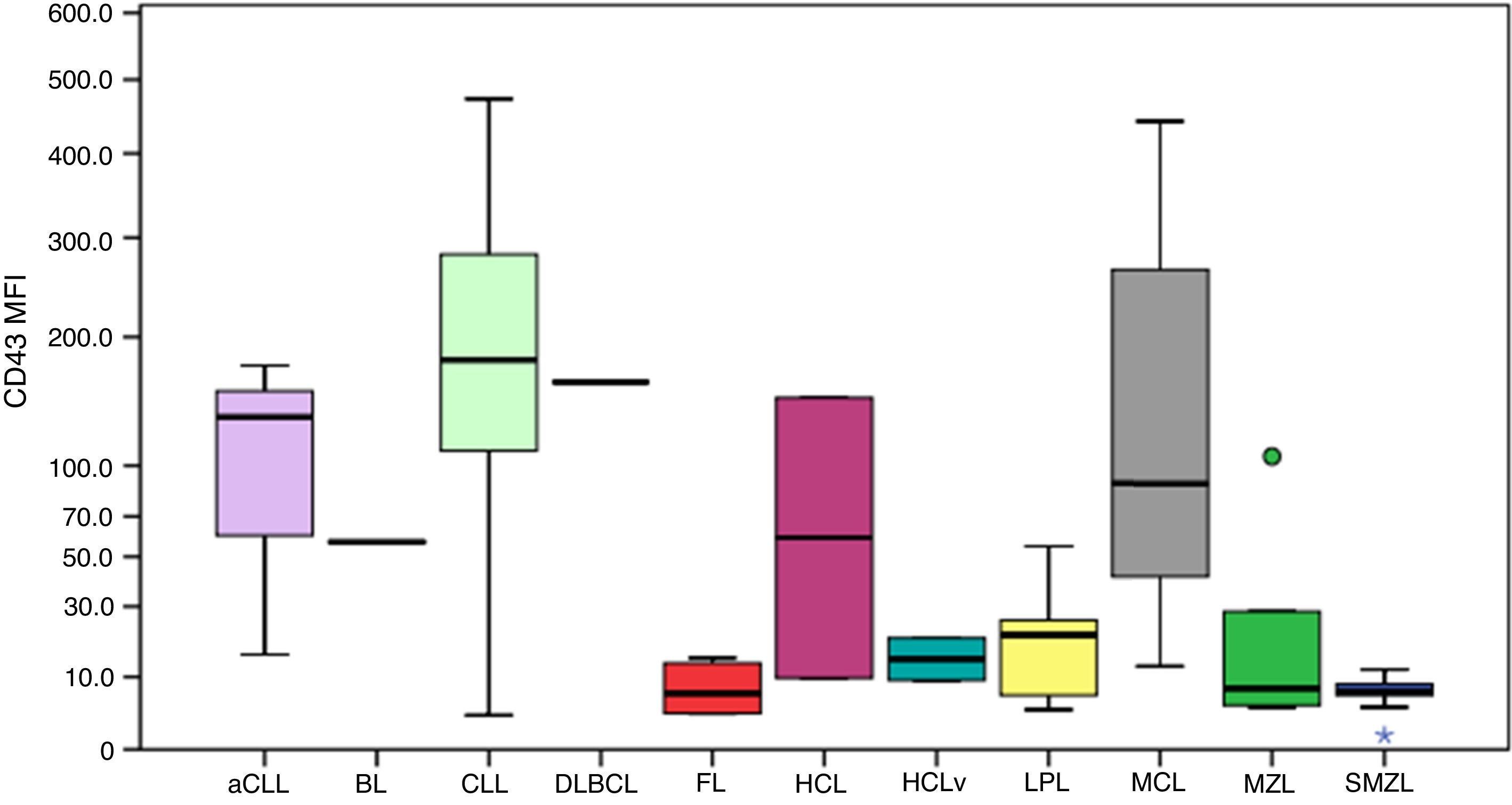
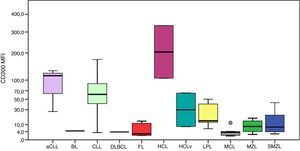
Figure 3 illustrates the expression of CD43. All follicular lymphoma cases had very low MFI values. The MFI of CD43 was higher in aCLL compared to MZL (p-value=0.034) and SMZL (p-value=0.010). The MFI of CD43 was higher in CLL compared to HCLv (p-value=0.021), LPL (p-value <0.001), follicular lymphoma (p-value=0.001), MZL (p-value <0.001) and SMZL (p-value=0.003).
Boxplot showing the MFI of CD43 in 124 cases of mature B-cell neoplasms. aCLL: atypical chronic lymphocytic leukemia; BL: Burkitt lymphoma; CLL: chronic lymphocytic leukemia; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; HCL: hairy cell leukemia; HCLv: hairy cell leukemia variant; LPL: lymphoplasmacytic lymphoma; MCL: mantle cell lymphoma; MZL: marginal zone lymphoma; SMZL: splenic marginal zone lymphoma. *One outlier case (MFI=1.1). One outlier case (MFI=106.3).
The MFI of CD43 was higher in MCL compared to LPL (p-value=0.013), follicular lymphoma (p-value=0.033), MZL (p-value=0.025) and SMZL (p-value=0.001).
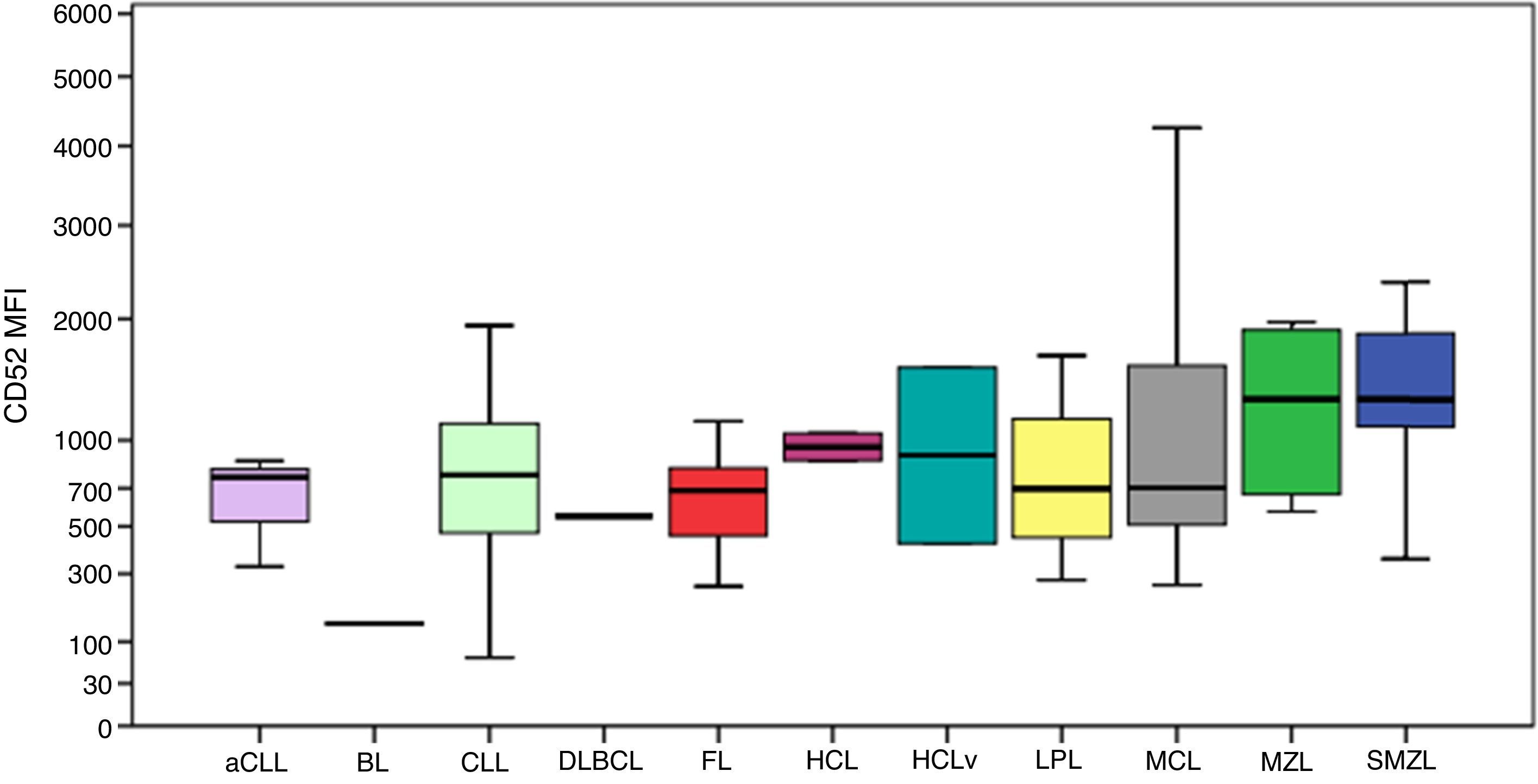
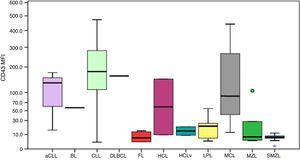
CD52 expression was consistently positive among all cases, as shown in Figure 4. The MFI of CD52 was higher in SMZL compared to CLL (p-value=0.001), aCLL (p-value=0.036), LPL (p-value=0.030) and follicular lymphoma (p-value=0.037). Despite the Burkitt lymphoma group only having one case, the MFI of CD52 seemed considerably lower compared to the remaining groups.
Boxplot showing the MFI of CD52 in 124 cases of mature B-cell neoplasms. aCLL: atypical chronic lymphocytic leukemia; BL: Burkitt lymphoma; CLL: chronic lymphocytic leukemia; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; HCL: hairy cell leukemia; HCLv: hairy cell leukemia variant; LPL: lymphoplasmacytic lymphoma; MCL: mantle cell lymphoma; MZL: marginal zone lymphoma; SMZL: splenic marginal zone lymphoma.
CD200 is a membrane glycoprotein of the immunoglobulin superfamily, expressed in dendritic cells, neurons and T-cell lymphocytes.8 Different research groups have identified the usefulness of this marker for the differential diagnosis of CLL versus MCL, including one analyzing a Brazilian population.9 The current study had similar results, and, although one CLL case showed low expression of this marker, 100% of MCL cases had significantly lower expressions compared to CLL. In a recent study of CLL patients, cases with low MFI of CD200 had a shorter time-to-treatment compared to patients with higher expression of the marker.10 As previously described in the immunohistochemical analysis by Pillai et al., CD200 can be extremely helpful in the differential diagnosis between HCL and HCLv11; in the present study, HCL cases showed a trend to have higher expressions than its variant form. There was no difference in CD200 expression between CLL and aCLL (CD5-negative) cases.
CD200 appears to be useful in the differential diagnosis between CD5-positive LPL and CLL, as it was lower in the former compared to the latter. However, if analyzed qualitatively, this difference could not have been noted, since 30% of cases showed intermediate positivity, which is in agreement with what was described in a previous study analyzing the expression of this marker by immunohistochemistry.12 CD200 has also been described as useful to differentiate between CLL and MZL; the results of the present study confirm the findings described by immunohistochemistry with a lower expression of the marker in MZL and SMZL.8
CD123 is a subunit of the interleukin-3 receptor. It has been described in several hematological neoplasms.13 Recently, CD123 expression was studied quantitatively using MFI by Garcia-Dabrio et al. who correlated its implication as a prognostic factor in de novo acute myeloid leukemia (AML),14 and SL-101, an anti-CD123 antibody-conjugate, is under study for the treatment of CD123-positive AML.15 The results of the current study are in agreement with previous studies investigating the higher expression of this marker in HCL,16,17 since higher MFI values were identified in HCL cases and a trend of HCL to have higher CD123 expression compared to HCLv cases was also observed. This study identified a higher expression of CD123 in SMZL compared to CLL, LPL and follicular lymphoma cases.
According to a previous study, CD43 expression is similar among CD10-positive MBCN (follicular lymphoma, Burkitt lymphoma and DLBCL), one of the most complicated differential diagnoses.18 All five cases of follicular lymphoma had extremely low expressions when compared to aCLL, CLL and MCL, and the only case of DLBCL expressed this marker at an intermediate intensity as described by previous studies,19 however, analysis of this group was impaired by the low frequency of cases.
During the ontogeny of B-cells, CD43 is expressed in early stages and is lost in intermediate stages, but it is expressed again in plasma cells and activated mature B-cells.20 In agreement with the literature, these results identified higher expression in aCLL, CLL and MCL, and lower expression in MZL and SMZL, probably due to clonality in the intermediate stage of the disease. Despite CD43 not being a relevant marker for the differential diagnosis of MCL and MZL, caution should be taken in the interpretation of these data with respect to the differential diagnosis of MCL versus CD5-positive MZL. One MCL case (16.0%) had a low expression of this marker similar to MZL and one MZL case (5.8%) showed intermediate expression similar to that seen in MCL.
CD52 is a cell surface glycoprotein whose function is poorly understood and is expressed in lymphocytes, monocytes, macrophages and a few dendritic cells.21 Its expression has been described in several MBCN and positivity for this marker involves specific treatments for diseases such as CLL and LPL.3,22,23 Furthermore, soluble CD52 has been identified as a marker of disease activity in CLL.24 This study identified the expression of CD52 in 100% of analyzed cases. Although these results seem to be in contrast with those described by other studies, such as Rodig et al. who reported negativity for some diseases such as Burkitt lymphoma and DLBCL,3 the lowest MFIs in the samples of the present study were exactly for Burkitt lymphoma and DLBCL. Chuang et al. analyzed MCL cases and identified that only 60% of cases were positive for CD52, but the difference in the immunohistochemical technique used may explain the distinct results.25
Since fluorescence intensity measures are important determinants in the analysis of leukemias and lymphomas,26 reliable methods for measuring MFI are important for the correct data interpretation, and, consequently, correct classification of hematological malignancies. For this reason, terms such as “weak” and “strong” are useful, but, as Henderson et al. suggested, perhaps quantitative values could be used in order to further explore the information provided.27
There are numerous variables involved in the quantitative determination of fluorescence intensity, some related to the specificity in the chosen MoAb, sample type, anticoagulant employed, autofluorescence, type of fixation, cytometer compensation and unit of measurement used to report the data, among others. Even when all these variables are well controlled, some caution in interpreting the data should be taken, but given the increasing universal standardization in the field of immunophenotyping, this type of analysis may gain ground, allowing for comparative figures with greater reliability over time. Until then, each center can develop the use of data in MFI according to their case series, in addition to using the data available in the literature, as long as it is critically interpreted and the limitations to this type of analysis are understood.
This study has some limitations. The main one is that only immunophenotyping and biopsy results as complementary tests for the disease entity definition were accessible. Besides, few cases of non-CLL cases, such as Burkitt lymphoma and DLBCL, were available.
ConclusionIn conclusion, the results of this study show the usefulness of already known markers in MBCN such as CD200 in the differential diagnosis of CLL and MCL. These data suggest the usefulness of more complex analyses quantifying MFI in the differential diagnosis of MBCN, such as CD52 expression in SMZL versus LPL, and CD43 expression in MZL, LPL and SMZL compared to MCL and CLL. Nevertheless, these results should be further explored in analyses using a larger sample size.
Conflicts of interestThe authors declare no conflicts of interest.
We acknowledge the Fundo de Incentivo a Pesquisa (FIPE) of Hospital de Clínicas de Porto Alegre (HCPA) for financial support.