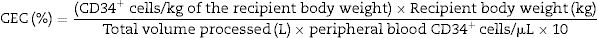An efficient mobilization and collection of peripheral blood stem cells (PBSCs) are crucial to optimize engraftment in the recipient. We aim to validate a formula that predicted CD34+ cell yield and to describe variables that correlated with high yield mobilization and collection in healthy donors.
MethodsWe retrospectively analyzed clinical and laboratory data from healthy donors who underwent PBSC collection from 2006 to 2015. The predicted number of collected cells was calculated using the following formula: Total number of CD34+ (cells×106/kg) yield=[(peripheral CD34+ cells/μL)×(0.43)/recipient body weight (kg)]×total liters processed.
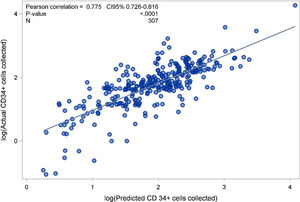
ResultsWe evaluated 338 collections from 307 allogeneic PBSC donors. The predicted versus the observed number of CD34+ cells/kg collected yielded an r-value of 0.775 (0.726–0.816; p<0.0001). Overall, 55.7% donors had an acceptable mobilization level. Donors with a body weight <67kg were less likely to yield a satisfactory CD34+ cell count (OR=0.44; 95% CI 0.24–0.81), while a white blood cell (WBC) count >40×109/L (OR=3.69; 2.11–6.46) and platelet count ≥200×109/L (OR=2.09; 1.26–3.47) on the day of collection predicted a good level of mobilization. Predictors of a CD34+ cell yield/kg of ≥4×106 with only one apheresis session were: circulating CD34+ cells/μL >40 (OR=16; 6.94–36.93), hemoglobin ≥14g/dL (OR=3.40; 1.53–7.57), WBC >40×109/L (OR=4.61; 2.10–10.10) on the first collection day, and a positive delta weight between donor and recipient (OR=3.10; 1.36–7.06).
ConclusionThe formula for predicting CD34+ cell yield is accurate and suggests the optimal length of time for successful leukapheresis. Validation of the predictors of successful mobilization will help to further refine PBSC leukapheresis procedures.
Mobilized peripheral blood stem cells (PBSCs) collection is used for 99% of autologous transplantations and 71% of procedures in an allogeneic setting.1 An efficient mobilization program is crucial for an adequate yield of CD34+ cells in the collection product and maximizes chances for an appropriate engraftment in the recipient. Depending on the policy of individual transplant centers, the type of transplant, and patient diagnosis, a minimum of 3–8×106 CD34+ cells/kg is necessary to perform the PBSC transplantation.2
Formulas for predicting CD34+ cell yield by leukapheresis have been proposed in the past few years. The predicted number of CD34+ cells can be calculated by the mathematical equation: Total number of CD34+ (cells/kg) yield=[(peripheral CD34+ cells/L)×(collection efficiency coefficient%)/recipient body weight (kg)]×total liters processed.3 The collection efficiency coefficient (CEC) varies from 30% to 50% depending on the automatic leukapheresis processor used, the skill of the collection staff and the venous access of the donor. In the original formula described above, the authors applied a CEC of 30% to guarantee the minimum number of CD34+ cells/kg that might be collected in a single day. The most commonly used system in Latin America is the COBE Spectra Apheresis System (Terumo BCT, Tokyo, Japan), which requires intermittent optical/manual input from the operator, making it labor-intensive and prone to user-dependent variability. The quality of cells collected with apheresis systems such as COBE has been reported to depend on the operator and a stable blood flow.4 The electronics-assisted Terumo Optia system has been largely used in Europe and the United States. Other systems, such as Amicus Fenwall and Com Tech Fenwall, are commonly used to collect PBSC, but are less popular.
The main predictor of a successful collection is the peripheral blood CD34+ cells count on the day of apheresis. Factors associated with a successful CD34+ cell mobilization in healthy donors have been reported. Bertani et al.5 in a retrospective multicenter study of 360 healthy donors identified a number of variables that correlated with PBSC mobilization yield. Male gender, younger donor age, elevated baseline white blood cell (WBC) count, higher doses of granulocyte-colony-stimulating factor (G-CSF) administration and the use of lenograstim instead of filgrastim were all associated with a good mobilization. Teipel et al.6 published the largest evaluation of predictors of good mobilization in healthy donors. In 7,216 unrelated allogeneic PBSC donors female sex, older age, smoking, elevation of lactate dehydrogenase, a higher relative lymphocyte count and an elevated large unstained cell count at baseline were negatively correlated with the CD34+ cells count on day +5 of mobilization. Conversely, a higher platelet, absolute lymphocyte and relative monocyte count, along with a higher body mass index at baseline was positively correlated with the CD34+ cells count on day +5. Billen et al.7 showed that female donors and donors who are of lower weight than their recipients were less likely to meet the CD34+ cell dose required for PBSCs transplantation. Ferritin levels,8 hematocrit (Htc) levels,9 race and ethnicity,10 and baseline peripheral blood CD34+ cells count11 have also been associated with mobilization outcome and collection of PBSC.
Studies evaluating predictors of successful PBSC collection in healthy donors are still scarce. Identification of relevant predictors and the ability to adequately estimate the CD34+ cell yield would improve collection strategies. The aim of the present study is to validate a formula to predict CD34+ cell yield and to describe predictors of successful collection among healthy donors at our institution.
MethodsDesign and settingWe retrospectively reviewed data from all healthy donors who underwent leukapheresis to collect PBSC from 2006 to 2015 at our institution. Fundação Pró-Sangue collects annually approximately 140,000 whole blood units, 4,800 platelets by apheresis, 40 granulocyte concentrate units by apheresis and 40 PBSC for stem cell allogeneic transplants. Recipients of allogeneic stem cell transplants are referred to the Bone Marrow Transplantation Team from the Department of Hematology and Department of Oncology of Hospital das Clínicas, the largest public hospital in Brazil. Allogeneic donors were selected according to Brazilian Ministry of Health policies, following a comprehensive medical examination and laboratory testing.
ProceduresA trained physician and/or nurse explained the procedures for PBSC collection and eligible donors signed an informed consent before starting G-CSF administration and also leukapheresis. Donors were mobilized exclusively with recombinant G-CSF, mostly in a single subcutaneous daily application for four to seven days. Dosing usually started as 10mcg/kg daily and later increased to a maximum of 20mcg/kg if mobilization was not adequate; for some unrelated donors, a starting dose of 5mcg/kg was used. As each G-CSF syringe had 300mcg, sometimes the dosing was rounded off. Peripheral CD34+ counting was performed on the fifth day after the start of G-CSF administration and, in the majority of cases; the first leukapheresis was also scheduled to start on that date. The processed volume was of 3–4 times the donor's total body volume for each procedure. If the required number of CD34+ cells/kg of recipient's body weight was not achieved, another collection was initiated the next day preceded by G-CSF administration in the morning. The collection of PBSC was performed with a continuous-flow cell separator (COBE Spectra Apheresis System, Terumo BCT, Tokyo, Japan), with a blood flow volume between 40 and 60mL and, on average, for 4–5h. We defined a successful PBSC collection when at least 4×106 CD34+ cells/kg were collected with only one apheresis session. We defined a good mobilization when the donor's peripheral circulating CD34+ cell count was at least 50/μL on the first day of collection.
Complete blood count (CBC) was performed on samples taken at two timepoints: immediately before apheresis, and after reinfusion at the end of the procedure. Hemoglobin (Hb) and Htc levels, WBC and platelet (PLT) counts were analyzed using the Sysmex XS 1000i automatic analyzer counter (Roche Diagnostics). CD34+ cell count in the peripheral blood and in the collected products were measured by flow cytometry, as described previously.12
We reviewed data from donors (gender, age, body weight, total blood volume, mobilization regimen), donors’ laboratory results (peripheral CD34+ cells, Hb, Htc, WBC and PLT counts) before and after leukapheresis, recipients’ data (body weight, age, diagnosis) and leukapheresis parameters for each procedure (processed blood volume, collected volume of the product, number of collected CD34+ cells/kg and collection efficiency coefficient [CEC]).
The formula applied to calculate CEC was:
The predicted number of collected cells was calculated using the formula previously described,3 applying the median CEC observed in the studied population. The formula used to predict the number of CD34+ cells yield in each collection was:
†Our median CEC% (in decimals) calculated in the studied population.
Statistical analysisUnadjusted and adjusted analyses were conducted by using logistic regression (LR) models to determine the association of covariates with binary outcome. Multivariate modeling was performed using Backward elimination (from a set of variables with p<0.15 in univariate LR analysis) with a significance level of 0.05 for a covariate to stay in model. Covariates with high percentages of missing values (more than 10%) were not included in the multiple logistic regressions. We evaluated multicolinearity by assessing the variance inflation factor (VIF).13 A VIF >2.5 for the logistic regression model was used as an indicator of multicolinearity.13 We assessed the model's discrimination and overall calibration using the c-statistic with 95% confidence intervals and the Hosmer–Lemeshow (H–L) goodness-of-fit test respectively. A p value of >0.05 as per the Hosmer–Lemeshow test indicates that the model is calibrated, i.e., the probabilities predicted by the model adequately reflect event occurrence. The following interpretation for c-statistic was considered: c=0.5, absence of discrimination; 0.5≤c<0.7, discrimination of little relevance; 0.7≤c<0.8, acceptable discrimination; 0.8≤c<0.9, excellent discrimination ≥0.9, near perfect discrimination. The continuous covariates were categorized based on published/clinically-relevant cut-offs or terciles of the distribution because the assumption of linearity in the logit was not supported. The assumption of linearity in the logit (log-odds) scale was evaluated using smoothed scatterplots.14 The logistic regression results were presented as odds ratios (OR) with Wald 95% confidence interval (CI) and p-values. Linear correlation was conducted according to Pearson's correlation coefficient. Where indicated, variables were logarithmically transformed (base e) to achieve normal distributions. The correlation coefficient was interpreted as follows: very strong linear correlation |r|=0.9–1.0; strong |r|=0.7–0.9; moderate |r|=0.4–0.7; weak |r|=0.2–0.4; very weak |r|=0.0–0.2. Simple linear regression was also applied. The assumptions of normality, homoscedasticity and independence of residuals were assessed graphically. The coefficient of determination, R2 was used as measures of the goodness of fit. Quantitative variables were reported as mean±standard deviation (SD) or median (inter-quartile range) when appropriate.15 Categorical variables were expressed as counts and percentages. Normality was assessed by visual inspection of histogram plots and use of D’Agostino-Pearson omnibus normality test. All statistical analyses were performed using SAS 9.3. A p-value of <0.05 was considered to be statistically significant, and all reported p-values were two-sided.
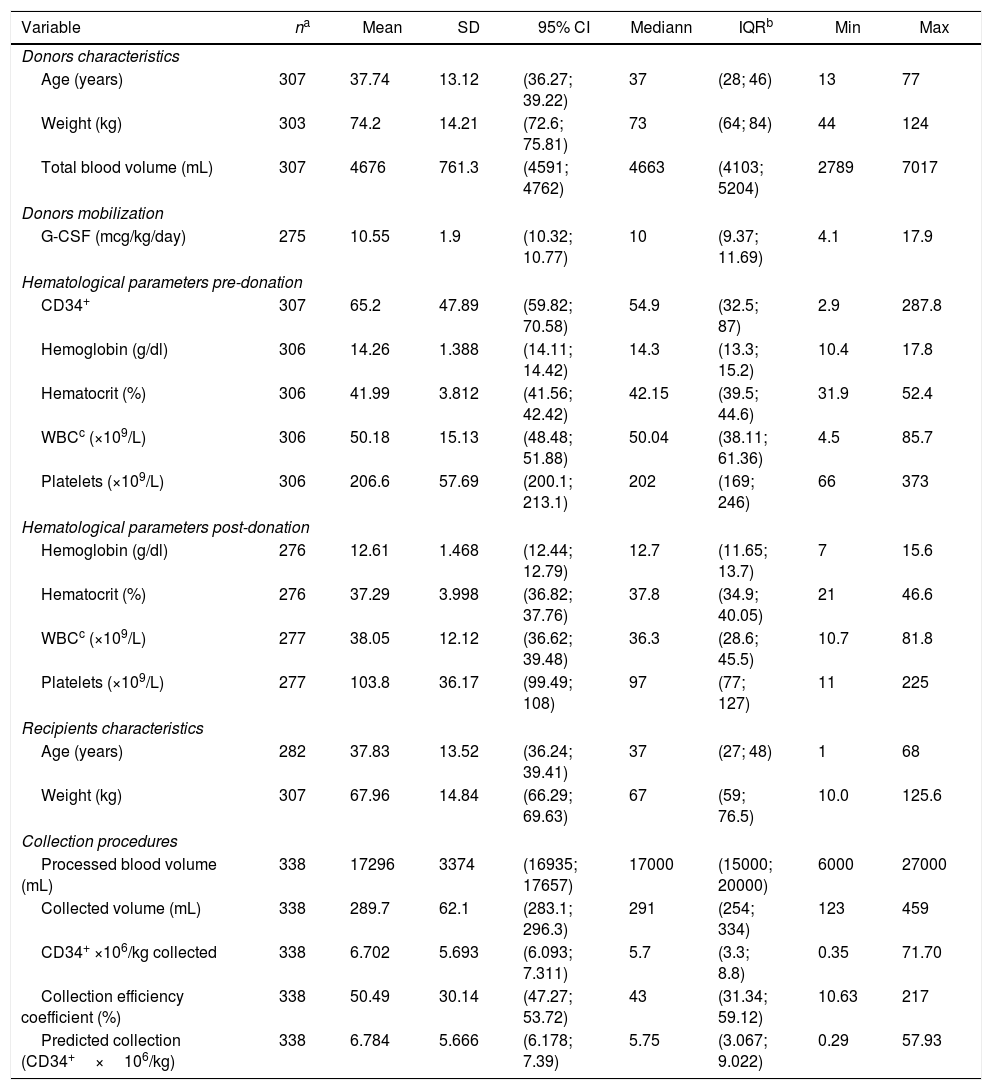
ResultsWe evaluated 338 collections from 307 allogeneic PBSC donors. In 279 (90.9%) donors we performed only one leukapheresis, in 26 (8.5%) donors two leukapheresis, in one donor (0.3%) three collections and in one (0.3%) four collections. Among them, 218 (71%) were men and the median inter-quartile range (IQR) values for age was 37 years-old (28; 46), for body weight was 73kg (64; 84), and for the total blood volume was 4,663mL (4103; 5204). Donors were mobilized with G-CSF with a median (IQR) dose of 10μg/kg/day (9.37; 11.69). The median (IQR) CBC values of the donors on the day of collection were: Hb level of 14.3g/dL (13.3; 15.2), WBC count of 50.04×109/L (38.11; 61.36), PLT count of 202×109/L (169; 246); the median (IQR) circulating CD34+ cells were 54.9/μL (32.5; 87). After collection, there were decreases in the median Hb level (11.2%), WBC count (28%) and PLT count (52%). Recipients’ median (IQR) for age and body weight were 37 years-old (27; 48) and 67kg (59; 76.5), respectively. Indications for PBSC transplantations were acute myelogenous leukemia (35.5%), acute lymphoblastic leukemia (17.6%), non-Hodgkin lymphoma (16.6%), chronic myelogenous leukemia (8.8%), myelodysplastic syndrome (8.4%), Hodgkin lymphoma (3.6%), myelofibrosis (3.6%) and others (12.3%).
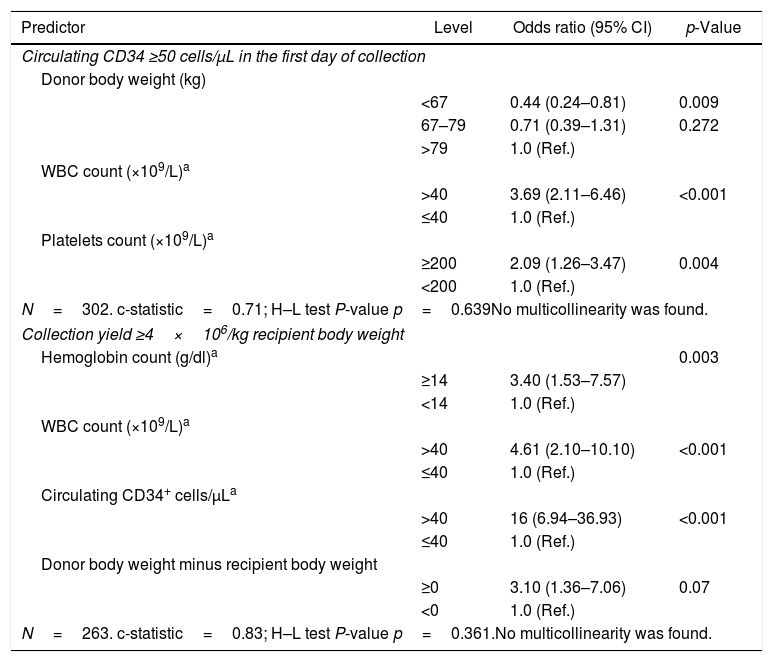
Overall, 171 (55.7%) donors presented with at least 50 circulating CD34+ cells/μL on the first day of collection. Among the donors, median (IQR) values on the first collection day were 36 years-old (28; 44), 75.5kg of body weight (66; 85), 4,758mL of total blood volume (4215; 5313), Hb of 14.4g/dL (13.6; 15.3), WBC count of 54.8×109/L (45.4; 65.5), PLT count of 215.5×109/L (179; 259), and 82.05/μL circulating CD34+ cells (63.8; 109.1). Univariate analysis showed that a body weight <67kg (p=0.011) was associated with a circulating CD34+ cells count <50/μL, whereas age between 31 and 44 years (p=0.026), WBC ≥40×109/L (p<0.001) and PLT count ≥200×109/L (p<0.01) were each associated with a circulating CD34+ cells count ≥50 cells/μl. Multivariate analysis showed that donors with body weight <67kg were less likely to have a good mobilization (OR=0.44; 95% CI 0.24–0.81). Conversely, a WBC count >40×109/L (OR=3.69; 95% CI 2.11–6.46) and PLT count ≥200×109/L (OR=2.09; 95% CI 1.26–3.47) on the first day of collection were each associated with a good mobilization (Table 2).
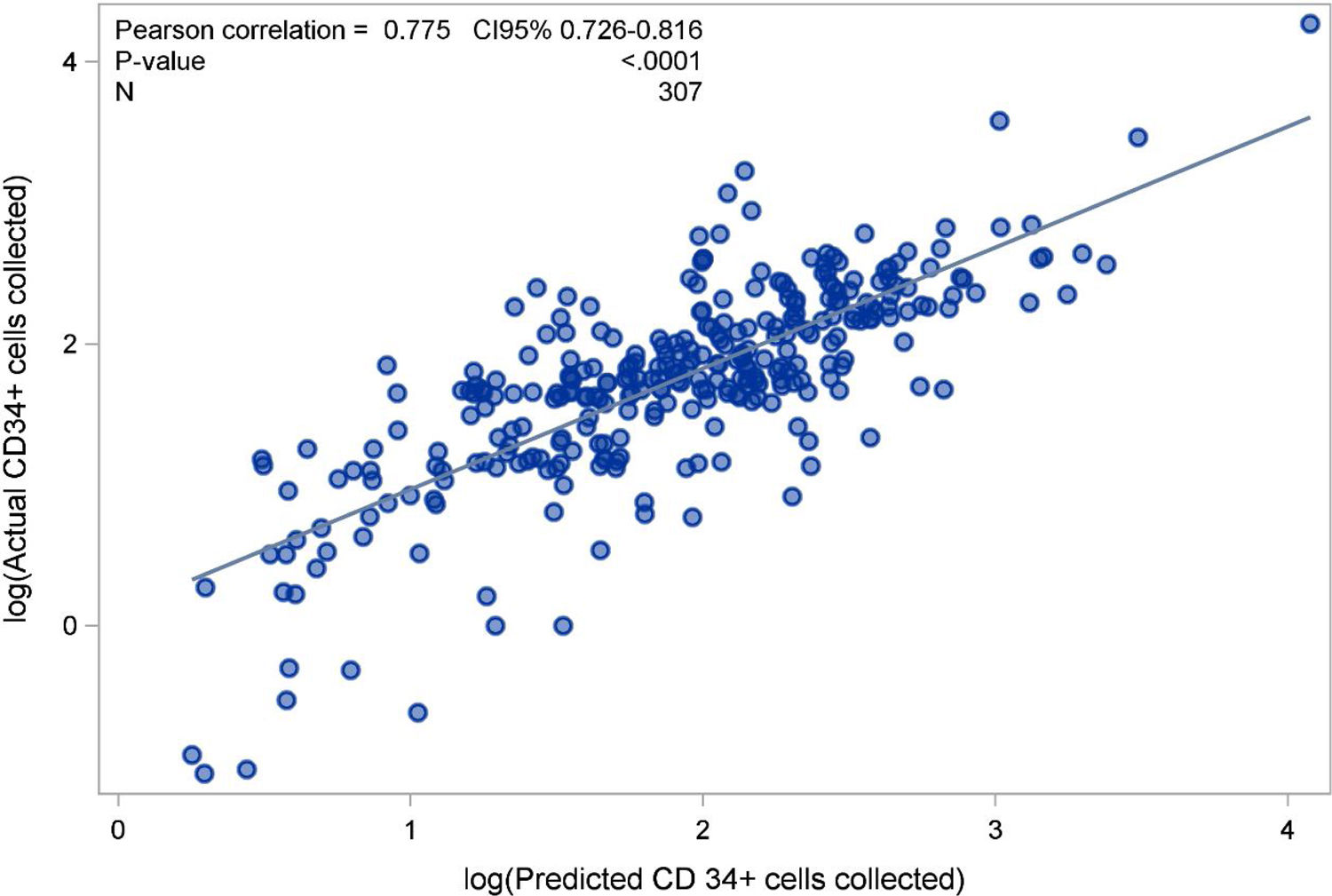
A minimum of 4×106 CD34+ cells/kg of the recipients’ body weight was harvested with only a single leukapheresis in 75.3% of the procedures. The median (IQR) for CD34+ cells×106/kg collected in the products was 5.70 (3.3; 8.8) and the median CEC was 43%. Considering the median CEC achieved, the collection predicting formula to calculate the total number of CD34+ cells collected by leukapheresis showed that the median (IQR) CD34+ cells×106/kg estimated to be collected in each product was 5.75 (3.067; 9.022), was very close to the actual median (IQR) amount of 5.7 (3.3; 8.8) (Table 1). Predicted versus observed data showed that the number of CD34+ cells/kg collected had an r-value of 0.775 (0.726–0.816; p<0.0001), demonstrating a linear correlation (Figure 1). Male donors (p=0.013), younger age (≤31 years, p=0.009; between 31 and 44 years, p=0.003), body weight >67kg (p=0.003), a positive difference in weight between donor and recipient (p=0.004), Hb level ≥14g/dl (p<0.001), PLT count ≥200×109/L (p=0.004), WBC >40×109/L (p<0.001) and circulating CD34+ cells >40/μL on the day of collection (p<0.001) were all associated with a successful collection in univariate analysis. In the multivariate model, predictors of collecting at least 4×106 CD34+ cells with only one collection were circulating CD34+ cells/μL >40 (OR=16; 95% CI 6.94–36.93), Hb level ≥14g/dl (OR=3.40; 95% CI 1.53–7.57), WBC count >40×109/L (OR=4.61; 95% CI 2.10–10.10) on the collection day, and a positive difference in weight between donor and recipient (OR=3.10; 95% CI 1.36–7.06) (Table 2).
Descriptive measures of 307 healthy donors’ and recipients’ characteristics, donors’ mobilization and hematologic blood counts, circulating CD34+ cells on the first day of collection in 338 collection procedures.
| Variable | na | Mean | SD | 95% CI | Mediann | IQRb | Min | Max |
|---|---|---|---|---|---|---|---|---|
| Donors characteristics | ||||||||
| Age (years) | 307 | 37.74 | 13.12 | (36.27; 39.22) | 37 | (28; 46) | 13 | 77 |
| Weight (kg) | 303 | 74.2 | 14.21 | (72.6; 75.81) | 73 | (64; 84) | 44 | 124 |
| Total blood volume (mL) | 307 | 4676 | 761.3 | (4591; 4762) | 4663 | (4103; 5204) | 2789 | 7017 |
| Donors mobilization | ||||||||
| G-CSF (mcg/kg/day) | 275 | 10.55 | 1.9 | (10.32; 10.77) | 10 | (9.37; 11.69) | 4.1 | 17.9 |
| Hematological parameters pre-donation | ||||||||
| CD34+ | 307 | 65.2 | 47.89 | (59.82; 70.58) | 54.9 | (32.5; 87) | 2.9 | 287.8 |
| Hemoglobin (g/dl) | 306 | 14.26 | 1.388 | (14.11; 14.42) | 14.3 | (13.3; 15.2) | 10.4 | 17.8 |
| Hematocrit (%) | 306 | 41.99 | 3.812 | (41.56; 42.42) | 42.15 | (39.5; 44.6) | 31.9 | 52.4 |
| WBCc (×109/L) | 306 | 50.18 | 15.13 | (48.48; 51.88) | 50.04 | (38.11; 61.36) | 4.5 | 85.7 |
| Platelets (×109/L) | 306 | 206.6 | 57.69 | (200.1; 213.1) | 202 | (169; 246) | 66 | 373 |
| Hematological parameters post-donation | ||||||||
| Hemoglobin (g/dl) | 276 | 12.61 | 1.468 | (12.44; 12.79) | 12.7 | (11.65; 13.7) | 7 | 15.6 |
| Hematocrit (%) | 276 | 37.29 | 3.998 | (36.82; 37.76) | 37.8 | (34.9; 40.05) | 21 | 46.6 |
| WBCc (×109/L) | 277 | 38.05 | 12.12 | (36.62; 39.48) | 36.3 | (28.6; 45.5) | 10.7 | 81.8 |
| Platelets (×109/L) | 277 | 103.8 | 36.17 | (99.49; 108) | 97 | (77; 127) | 11 | 225 |
| Recipients characteristics | ||||||||
| Age (years) | 282 | 37.83 | 13.52 | (36.24; 39.41) | 37 | (27; 48) | 1 | 68 |
| Weight (kg) | 307 | 67.96 | 14.84 | (66.29; 69.63) | 67 | (59; 76.5) | 10.0 | 125.6 |
| Collection procedures | ||||||||
| Processed blood volume (mL) | 338 | 17296 | 3374 | (16935; 17657) | 17000 | (15000; 20000) | 6000 | 27000 |
| Collected volume (mL) | 338 | 289.7 | 62.1 | (283.1; 296.3) | 291 | (254; 334) | 123 | 459 |
| CD34+ ×106/kg collected | 338 | 6.702 | 5.693 | (6.093; 7.311) | 5.7 | (3.3; 8.8) | 0.35 | 71.70 |
| Collection efficiency coefficient (%) | 338 | 50.49 | 30.14 | (47.27; 53.72) | 43 | (31.34; 59.12) | 10.63 | 217 |
| Predicted collection (CD34+×106/kg) | 338 | 6.784 | 5.666 | (6.178; 7.39) | 5.75 | (3.067; 9.022) | 0.29 | 57.93 |
Predictors of good mobilization of peripheral blood stem cells (PBSCs) and successful collection of PBSC among 307 healthy donors.
| Predictor | Level | Odds ratio (95% CI) | p-Value |
|---|---|---|---|
| Circulating CD34 ≥50 cells/μL in the first day of collection | |||
| Donor body weight (kg) | |||
| <67 | 0.44 (0.24–0.81) | 0.009 | |
| 67–79 | 0.71 (0.39–1.31) | 0.272 | |
| >79 | 1.0 (Ref.) | ||
| WBC count (×109/L)a | |||
| >40 | 3.69 (2.11–6.46) | <0.001 | |
| ≤40 | 1.0 (Ref.) | ||
| Platelets count (×109/L)a | |||
| ≥200 | 2.09 (1.26–3.47) | 0.004 | |
| <200 | 1.0 (Ref.) | ||
| N=302. c-statistic=0.71; H–L test P-value p=0.639No multicollinearity was found. | |||
| Collection yield ≥4×106/kg recipient body weight | |||
| Hemoglobin count (g/dl)a | 0.003 | ||
| ≥14 | 3.40 (1.53–7.57) | ||
| <14 | 1.0 (Ref.) | ||
| WBC count (×109/L)a | |||
| >40 | 4.61 (2.10–10.10) | <0.001 | |
| ≤40 | 1.0 (Ref.) | ||
| Circulating CD34+ cells/μLa | |||
| >40 | 16 (6.94–36.93) | <0.001 | |
| ≤40 | 1.0 (Ref.) | ||
| Donor body weight minus recipient body weight | |||
| ≥0 | 3.10 (1.36–7.06) | 0.07 | |
| <0 | 1.0 (Ref.) | ||
| N=263. c-statistic=0.83; H–L test P-value p=0.361.No multicollinearity was found. | |||
The number of CD34+ cells predicted by the formula and the actual number of CD34+ cells collected showed a strong correlation. Review of each PBSC collection and systematic calculation of the individual CEC for each procedure can be helpful in more precisely predicting the number of CD34+ cells that will be collected. A difference from other reports is that in the present study the predicting collection formula was validated exclusively from healthy donors. The number of circulating CD34+ cells on the day of leukapheresis, as broadly reported, was the strongest predictor of a successful collection and depended on a good mobilization. Additionally, a higher Hb level, higher WBC count and a positive delta weight between donor and recipient were also predictors of a successful PBSC collection. We found that higher WBC and PLT counts immediately before leukapheresis were associated with a good mobilization, while donors with low body weight were more likely to be poor mobilizers.
The first validated formula to predict collection of CD34+ cells by leukapheresis included only 9 healthy donors and the adjusted Terumo COBE Spectra CEC was 30% to guarantee at least the minimum number of CD34+ cells/kg that might be expected to be collected in a given day for any donor.12 Further, a similar predicting collection formula was validated only among autologous PBSC collections using a CEC of 40%.16 In 2014, Hosing at al.17 assessed the predictive value of the above-mentioned formula in 1,126 consecutive PBSC collections, 44 of which were from healthy donors. In our series, in contrast to other reports, all collections were performed on healthy allogeneic donors and we used the median CEC achieved in the collections to validate the efficacy of the formula. CEC depends on the collection processor, the skill of the collection team and the accumulated experience acquired in each service. We consider that using the historical CEC series in each procedure can improve the ability of PBSC collection services to more accurately predict the actual amount of CD34+ cells to be obtained by leukapheresis.
The identification of risk factors for poor PBSC mobilization and collection among healthy donors is essential to improve donor selection and strategies to ensure the most efficient recipient engraftment. A hematopoietic cell transplantation modality that is recently gaining importance is haploidentical transplantation. This modality of transplant requires higher amounts of stem cells to overcome the HLA barrier18 and, consequently, demands a selection of a “good mobilizer” donor and a specific leukapheresis schedule to optimize collection in large volume apheresis.
The G-CSF dose is calculated using the actual body weight and not the ideal body weight. Consequently, heavier donors receive a higher G-CSF dose and higher WBC counts can be found before leukapheresis. In line with this protocol, lower body weight was found to be negatively related with the CD34+ cells count on the collection day.6 Murine experiments showed that adipose tissue has several structural similarities with bone marrow and contains significant amounts of stem cells19 that can be mobilized into the bloodstream. Further investigation is needed to assess if these findings are similar and relevant for humans. Additionally, CBC on the day of collection is a simple and inexpensive test that is routinely performed as part of the collection process. WBC count and Hb levels are closely related with the overall hematopoiesis20 and can be used as a sensitive surrogate marker of successful collection.
Donor selection is primarily dependent on HLA compatibility and characteristics such as age, gender, and body weight. The preferential recruitment of male donors has been adopted by many services, considering that women and donors who are of lower weight than their recipients have a decreased likelihood of meeting the transplant physicians’ requested dose.6 A positive delta body weight between donor and recipient is an efficient predictor of successful collection and should be taken into consideration in selection of PBSC donors, especially when elevated amounts of CD34+ cells are needed.
There are some limitations to our study. Different from other studies4,5,7,21–23 we could not evaluate if there is an association between gender and poor mobilization or unsuccessful PBSC collection since most of the participants were men. We also did not collect data on the baseline CBC, before the first G-CSF dose, limiting the identification of other potential predictors of a good mobilization. However, we collected data from a consecutive group of PBSC healthy donors over a 10 year period. This group is representative of the current donors’ selection practices at our institution. Furthermore, our collection data cannot be extrapolated to other apheresis equipment. In the last year, new apheresis equipment, which achieves a higher CEC and a more efficient PBSC collection, has been launched. COBE Spectra is still the leading brand in Latin America and other parts of the world. This popularity reinforces the utility of our results to other collection services.
In conclusion, the described formula for predicting CD34+ cells yield is useful to determine the required length of the procedure, and whether a second day is likely to be necessary. An accurate prediction of the leukapheresis length also save donors’ time. Predictors identified have value to the refinement of PBSC leukapheresis procedures, optimizing human and material resources. Further studies to better identify predictors of poor mobilization will further help to select donors that may benefit from different regimens, for example in combination with plerixafor. Recent studies reported the use of plerixafor, off-label, in healthy family donors as a rescue strategy to achieve an appropriate amount of CD34+ cells and provide an adequate graft for PBSC transplantation.24–26 Finally, on-site computer applications to accurately achieve a successful PBSC yield can be assembled and prospectively validated in other populations using both the formula to predict PBSC yield and the predictors described herein.
FundingNone declared.
Conflicts of interestThe authors declare no conflicts of interest.
All authors fulfill the following three criteria: substantial contributions to the research design or the acquisition, analysis or interpretation of data; drafting the paper or revising it critically; and approval of the submitted and final versions.
C.A.N. and A.M.J. designed the research study, performed the research, analyzed data, wrote the manuscript and reviewed and edited text and tables. D.T.H., A.M.A. and M.C.F. collected data, analyzed data and reviewed text, tables and figure. F.R.M. analyzed data and reviewed and edited text and tables. S.S.W. and V.R. reviewed and edited the text, approved the final version.
We would like to thank Ms. Susana Alves dos Santos and Mr. Diogo Costa de Freitas, administrative assistants of Fundação Pró-Sangue Hemocentro de São Paulo, who kindly helped to retrieve all the study participants’ registries.