Abnormal coagulation tests are frequently observed in critically ill patients even in the absence of haemorrhagic symptoms. In patients with severe COVID-19 abnormal coagulation tests, including an isolated prolonged APTT have been reported in patients with severe Covid-19.1 These include increased levels of D-Dimero, fibrinogen, FVIII and Factor von Willebrand, and increased prothrombin (PT) and activated partial thromboplastin (APTT) times.1 Infection with the SARS-CoV-2 virus can cause a hyper-coagulable state known as COVID-19 associated coagulopathy (CIC) and is associated with both venous and arterial thrombosis. As such prophylactic anticoagulation with heparin or low molecular weight heparin is recommended in all hospitalized patients who have no contraindications.2
However a prolonged APTT in these patients may result in inadequate or no antithrombotic therapy, the use inappropriate use of blood products such as fresh frozen plasma and delay in invasive procedures or surgery. A prolonged APTT may indicate the deficiency of a coagulation factor, the factors VIII, IX, XI, XII and von Willebrand disease or the presence of an inhibitor which may be specific e.g. anti-Factor VIII or non-specific such as the anti phospholipid syndrome.3 A prolonged APTT should prompt further investigation especially in patients without prior anticoagulation therapy or liver disease. The presence of an elevated haematocrit (>55%), incomplete filling of the sample tube, contamination with heparin or prolonged time between the sample collection and processing (> 4 hours) all may lead to a falsely prolonged APTT and should be excluded.4 If these causes are excluded a mixing study with normal plasma in a 1:1 and 1:4 ratio and the APTT performed immediately and after two hours incubation at 37ªC. An impaired correction of the APTT in either ratio or assessment is consistent with the presence of an inhibitor while the correction in all is consistent with a deficiency of one or more coagulation factors. The specific tests for an inhibitor or factor deficiency should then be performed to determine the specific abnormality.
We present the case of a 23 year-old man with moderately severe COVID-19 pneumonia and a prolonged APTT, the subsequent investigation and management.
Clinical caseA 23 year-old man presented to the emergency service with fever, a productive cough and dyspnoea. A thoracic CT scan was consistent with infection but not characteristic of COVID-19, a thoracic angio-CT and Doppler ultrasound of the lower limbs were negative for thrombosis. There was no clinical evidence of haemorrhage or bruising and arterial blood gases showed a p02 of 75 mm Hg (inferior limit of normal) and normal pC02. RT-PCR testing for SARS-CoV-2 was positive. The patient had no personal or family history of thrombosis or coagulopathy, a minor surgery 4 months earlier was uneventful and routine blood tests at this time, including PT and APTT were normal.
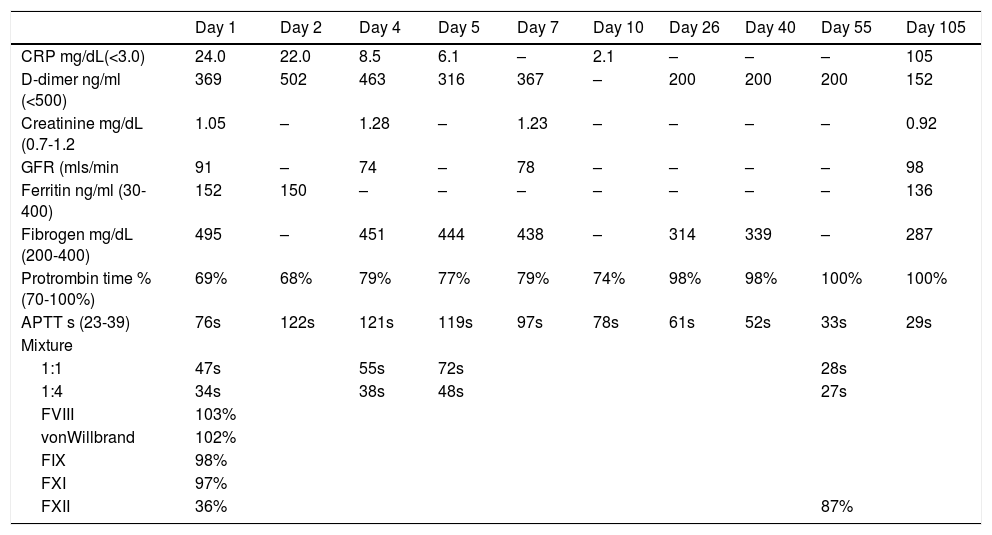
The patient had an elevated C reactive protein of 24 mg/L (normal <3 mg/L), a D-dimer of 369 ng/ml (normal), ferritin 152 ng/ml (normal) and fibrinogen of 495 mg/dL) (normal 200–400). The full blood count was normal, a PT of 69% (normal 70–100%) and an APTT of 76s (normal 23-39s). Mixtures with normal plasma and incubated for 2 h at 37°C showed a partial correction and 1:4 a full correction. Tests for anti-cardiolipins IgG and IgA, anti beta-2-glicoprotein IgG and IgA and lupus anticoagulant (dilute Russel´s viper-venom time) were all negative, levels of Factor VIII, IX, XI and von-Willebrand were all in the normal range (103%, 98%, 97% and 102% respectively). The level of FXII was decreased at 36% (normal range 55–160%) and a modified Bethseda assay showed an inhibitor of <5IU for Factor XII.
Treatment was supportive with oxygen; prophylaxis for venous thrombosis with enoxaparin was started and the patient did not require mechanical ventilation. The APTT subsequently increased and the mixture with normal plasma 1:1 corrected less (heparinise was used to correct any effect of heparin on the APTT), even though the inflammatory parameters decreased and the patient clinically improved (Table 1). The patient was discharge home on day 7 and followed-up in outpatients. At day 55 the APTT had returned to normal with a value of 33s, at day 105, the APTT and results of mixing with normal plasma were normal, and the FXII level had increased to 86% and no inhibitor was detected.
Laboratory findings from Day 1 until Day 55.
CRP: C reactive protein; GFR: glomerular filtration rate; APTT: activated partial thromboplastin time.
Factor XII is a single chain glycoprotein, present in plasma as the zymogen of serine protease Factor XIIa. It plays a role in the initiation of the intrinsic coagulation cascade and fibrin formation. Congenital deficiency of Factor XII is a rare autosomal recessive disorder with an incidence of approximately 1/106 individuals. Deficiency of Factor XII results in a marked prolongation of the APTT but is not associating with a haemorrhagic tendency. Its association with a thrombotic tendency is still debatable, it has been reported that the incidence of deep vein thrombosis in homozygous Factor XII deficiency is of the order of 11%, while in heterozygous patients thrombosis occurred in 2% of patients.5 Acquired inhibitors to FXII have been described, but only as sporadic cases. It has been associated with liver and gastrointestinal disease and remission of the underlying disease usually supressed the inhibitor production.6
Although a deficiency of Factor XII is frequent in critically ill patients with sepsis, with up to 50% having a prolonged APTT,7 the presence of an inhibitor is not. In patients with severe COVID-19 20% a cohort of 216 patients were reported to have a prolonged APTT of whom 91% were positive for the lupus anticoagulant.8 In this same cohort, deficiency of FXII of <50IU/dl were found in 7% of patients.8 An association between lupus anticoagulants and acquired factor XII deficiency secondary to factor XII antibodies has been described previously in patients with systemic lupus eritrematosis.8 In this reported case the lupus anticoagulant was not detected. In the context of COVID-19, other acquired deficiencies of coagulation factors have been reported, acquired FVIII9 and FXI.10 We speculate that the FXII inhibitor was produced in the setting of immune dysregulation from the underlying SARS-CoV-2 infection due to the absence of a prolonged APTT 4 months prior to the hospitalization for COVID-19. The reason for the production of inhibitors in the context of COVID-19 is not clear. FXII is not only a component of the coagulation system but participates in the process of contact activation, together with Factor XI, the bradykinin-generating kallikrein-kinin system and initial host defense mechanisms against infection. Part of the initial response to viral infections is mediated by contact activation, where both FXII and FXI appear to have a pivotal role. It is possible to speculate that decreased levels of FXII counterbalenced the state of hypercoagulability anfd inflammation, the so-called thromboinflammation caused by SARS-CoV-2 resulting in a milder disease.
The key points in this case, is that the acquired FXII deficiency was found in a patient with moderately severe COVID-19 who did not require mechanical ventilation or be admitted to the ICU. The presence of a prolonged APTT persisted long after the patient was clinically well, the inflammatory parameters had returned to normal and the patient discharged from hospital. The APTT which was normal four months prior to the admission had returned to normal at day 55 and day 105. At day 105 the FXII level was in the normal range and both mixing with normal plasma and the Bethseda assay were negative for an inhibitor. This confirmed the transitory nature of the inhibitor.
Levels of Factor XII lower than 42% are associated with a prolonged APTT but not with an increased risk of haemorrhage. It is important to determine the cause of a prolonged APTT so as not to omit thromboprophylaxis or anticoagulant treatment in patients with an increased risk of thrombosis. In a complex system such as the coagulation an APTT cannot be interpreted without reservation and needs to be investigated.
Even in patients with non-severe Covid-19 these abnormalities may be present and persist long after the patient is clinically well.
ConclusionsThis case report demonstrates the importance of investigating alternative causes of a prolonged APTT in patients with COVID-19. Although COVID-19 is associated with thrombotic complications it is possible that immune dys-regulation associated with SARS-CoV-2 infection may lead to other coagulation factor inhibitors and a haemorrhagic tendency.
Clinical decisions on anticoagulant treatment in these cases may be influenced by the APTT and modified in patients with Covid-19. Management of these cases requires the clinical acumen to balance the potential for haemorrhagic and thrombotic complications of SARS-CoV-2 infection. The abnormalities in the coagulation tests may persist long after clinical remission and resolution of inflammatory markers.