While first-line induction therapy for patients with multiple myeloma has changed over the years, autologous hematopoietic stem cell transplantation still plays a significant role, improving both depth of response and progression-free survival of myeloma patients. Our 25-year experience in mobilizing hematopoietic stem and progenitor cells for 472 transplant-eligible myeloma patients was retrospectively reviewed. Patients were stratified according to the remission induction therapy received, and the outcomes were compared among the cohorts that received vincristine, adriamycin and dexamethasone (VAD) (n=232), bortezomib and dexamethasone (BD) (n=86), cyclophosphamide, bortezomib and dexamethasone (CyBorD) (n=82) and other regimens (n=67). Cyclophosphamide plus granulocyte colony-stimulating factor was the predominant mobilization regimen given. A greater number of CD34+ cells (9.9×10E6/kg, p=0.026) was collected with less hospital admissions in BD patients (13%, p=0.001), when compared to those receiving VAD (7.5×10E6/kg, 29%), CyBorD (7.6×10E6/kg, 19%), or other regimens (7.9×10E6/kg, 36%). Induction therapy did not influence the overall rate of unscheduled visits or the length of hospitalization because of complications following mobilization. The myeloma response was not significantly deepened following the cyclophosphamide administered for mobilization. This analysis demonstrates the importance of monitoring the impact of initial treatment on downstream procedures such as stem cell mobilization and collection.
Multiple myeloma (MM) therapy has evolved significantly over the last two decades. As novel agents entered clinical practice, first-line treatment of transplant-eligible MM patients changed from MP (melphalan and prednisone) or VAD (vincristine, adriamycin, and dexamethasone) to regimens based on immunomodulators and proteasome inhibitors. Despite the positive impact of newer agents on response and survival, MM remains incurable and standard care still incorporates a consolidative autologous stem cell transplant (aHSCT) for deeper response and better progression-free survival (PFS).1–4
The collection of sufficient numbers of hematopoietic stem and progenitor cells (HSPC) is necessary for rapid and sustained engraftment following aHSCT. The definition of a successful collection varies among transplant centres, but it is frequently defined as a minimal HSPC dose of 2×106CD34+cells/kg for a single aHSCT.5 Because patients eventually relapse, a second aHSCT is a potential treatment option and the collection of adequate CD34+ cells for two transplants is commonly performed.6 Since HSPC collection can be affected by the myelotoxicity from induction regimens, it is important to recognize their effect on HSPC mobilization and collection outcomes. While melphalan and lenalidomide have been associated with collection failure and a lower cell yield,7–11 bortezomib does not appear to negatively affect HSPC collection and has been studied as a mobilization agent.12
Stem cell mobilization is not an innocuous procedure and can result in substantial morbidity. The frequency and severity of toxicity following mobilization is dependent on, but not limited to, which mobilizing agents are used.2,11,13,14 The granulocyte colony-stimulation factor (GCSF) is the most commonly used mobilizing agent. It can be used alone or in combination with plerixafor or chemotherapy, usually cyclophosphamide (CTX-GCSF). Mobilization with GCSF can cause bone pain, headache and rarely thrombotic events or splenic rupture. The addition of cyclophosphamide (CTX) to the mobilization regimen improves the HSPC harvest and might add an anti-myeloma effect, but at the cost of higher toxicity, when compared to patients mobilized with GCSF alone.1,15 Patients receiving CTX may experience febrile neutropenia, nausea, vomiting and severe hyponatremia.13,16 Plerixafor, recently added to the armamentarium of mobilizing agents, mobilizes sufficient HSPC in fewer aphereses with little or no additional toxicity when used with GCSF, compared to GCSF alone.17,18 This combination has become an alternative for patients who initially fail to mobilize sufficient CD34+ cells.19
Consecutive patients were treated with different induction chemotherapy based on contemporary guidelines of the Blood and Marrow Transplant program (BMT) at The Ottawa Hospital (TOH). Recognizing the changing landscape of induction therapy, we report our experience with the mobilization of HSPC in a large single-centre cohort of patients with myeloma and compare the impact of MM induction regimens used on the subsequent toxicity and efficiency of HSPC collection.
MethodsStudy designThis is a single-centre retrospective study of consecutive MM patients who were seen by the BMT program for HSPC collection for a first-line consolidative aHSCT between January 1991 and September 2015. Patients were excluded if their first HSPC collection was attempted outside TOH or occurred following salvage therapy. The details of patient selection are shown in supplemental figure S1. The study was approved by the local research ethics board.
The data was retrospectively extracted from office charts, hospital electronic medical records and the BMT database. Information collected included patients’ demographic data and their disease description comprising baseline characteristics, therapy and treatment outcomes. Cytogenetics and FISH were not routinely performed during the study period and therefore were not collected.
DefinitionsThe myeloma subtype was classified according to the secreted paraprotein heavy chain (IgG, IgA, IgD, IgM, or IgE) and light chain (kappa or lambda), or their absence (non-secretory). Patients were staged by the Salmon–Durie (SD) criteria and by the International Staging System (ISS).
Responses were defined by the International Myeloma Working Group (IMWG) criteria for serum (SPEP) and urine (UPEP) electrophoresis and immunofixation (IFE). We did not have access to serum free light chain assays until 2016 and therefore response was defined by SPEP, UPEP and IFE alone. Changes in the paraprotein were compared between the diagnosis and the last measurement before mobilization chemotherapy (induction response) and between the diagnosis and the last measurement before aHSCT (mobilization response). Responses were categorized as follows: complete response (CR) (patients with negative serum and urine IFE); very good partial response (VGPR) (detectable abnormal bands only by IFE or 90–99% monoclonal peak reduction in SPEP and <0.1g/24h of urinary paraprotein); partial response (PR) (50–89% paraprotein reduction in SPEP and ≥90% drop in UPEP); stable disease (SD) (<50% paraprotein reduction in SPEP and <25% paraprotein increase in SPEP or <90% paraprotein reduction in UPEP), and; progressive disease (PD) (≥25% paraprotein increase in SPEP or UPEP).
Toxicity was defined as an unscheduled medical visit to an emergency room or medical day care unit during the follow-up period after mobilization had been initiated. The reason for an unscheduled medical visit, need for admission, length of admission, and number of days spent in the intensive care unit (ICU) were collected. Infections included febrile neutropenia, non-neutropenic fever, skin infections or pneumonia after mobilization.
Neutrophil engraftment was defined as the first of 3 consecutive days with absolute neutrophil count ≥0.5×109/L and platelet engraftment was the first of 3 consecutive days with platelet count ≥20×109/L in the absence of platelet transfusion in the previous 7 days.
Remission inductionThe most common regimens administered, VAD, BD (bortezomib and dexamethasone) and CyBorD (CTX, bortezomib, and dexamethasone) were analyzed individually, whereas smaller groups (single agent dexamethasone, melphalan, thalidomide, and lenalidomide) and patients who received two different induction regimens were grouped together. The details of VAD, BD, and CyBorD regimens are outlined in supplemental Table S1.
Mobilization and collectionPatients mobilized with GCSF alone often received 5μg/kg/day of filgrastim subcutaneously for 4 days and proceeded to apheresis on day 5 if peripheral CD34+ assessment showed adequate HSPC mobilization. In CTX-GCSF mobilization, 1.5–4.5g/m2 of CTX was given, followed by filgrastim two days later until the last day of apheresis, as needed.
The target for HSPC collection was ≥2×106CD34+cells/kg, but the decision to proceed with aHSCT with <2×106CD34+cells/kg was made individually. Patients who did not mobilize sufficient HSPC were offered salvage plerixafor (0.24mg/kg) beginning in 2012.
We looked at the total CD34+ cells collected, and how many aphereses were required for a successful collection. The number of CD34+ cells re-infused was used when the number of cells collected was missing (n=56) because the patient had been treated before 2004, in an era when storing CD34+ cells for a second transplant was not routine practice in our centre.
Conditioning to aHSCTConditioning regimens varied during the study period and are described in supplemental Table 1.
Statistical analysisDescriptive statistics were used to report demographic, disease and treatment characteristics. Non-normal data was presented as medians with the range. Categorical data was compared using Pearson's chi-square test or Fisher's exact test, as appropriate. The analysis of variance (ANOVA) and t-tests were used to compare continuous variables and statistical significance was assigned when p<0.05. Statistical analyses were performed using the GraphPad Prism version 6.00.
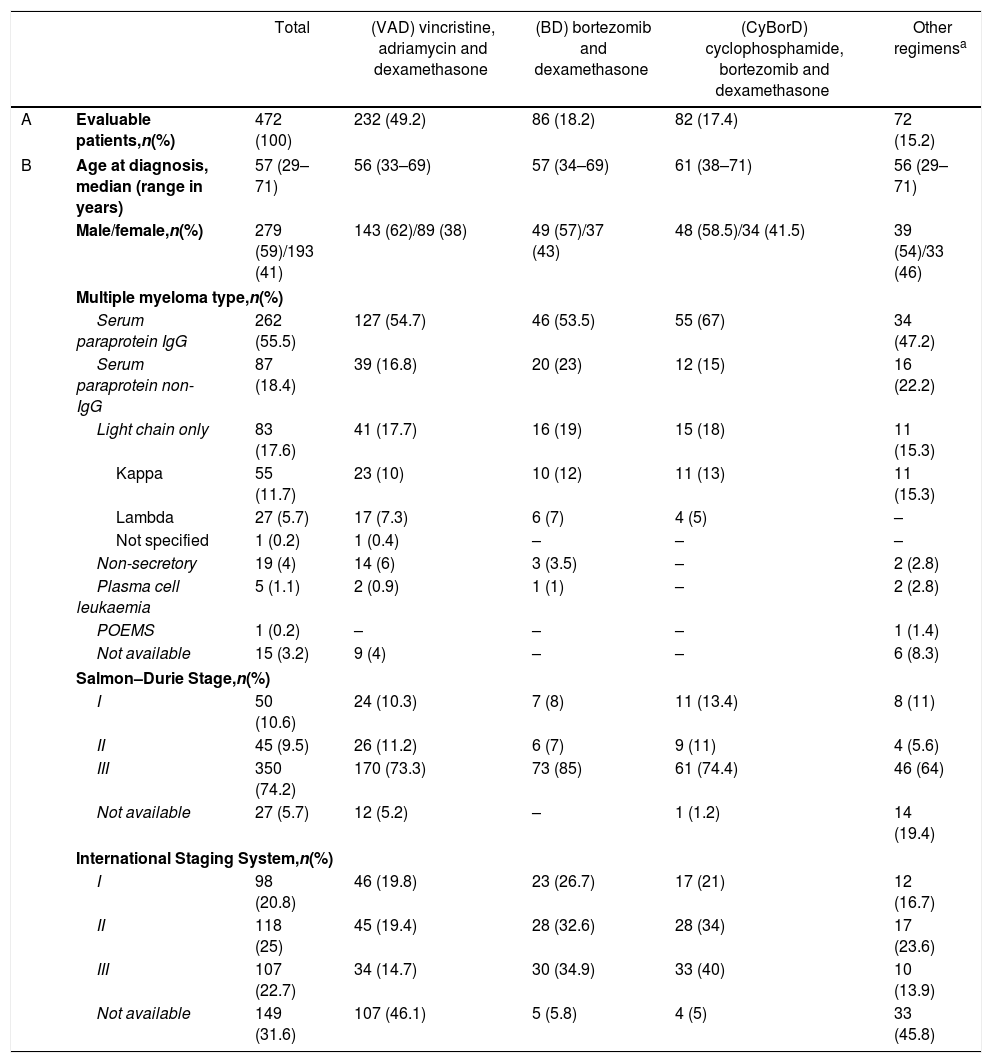
ResultsPatients and disease characteristicsFive hundred and ten consecutive MM patients were referred for a first-line HSPC mobilization. Thirty-eight patients were excluded, as their HSPC mobilization had not been part of the initial treatment (n=20) or because they had received a first-line allogeneic HSCT instead (n=18), leaving 472 patients in the study. Patient and disease baseline characteristics were similar among patients who had received a different induction therapy, except for the increased age seen in CyBorD patients (Table 1).
Patient and diagnosis characteristics.
| Total | (VAD) vincristine, adriamycin and dexamethasone | (BD) bortezomib and dexamethasone | (CyBorD) cyclophosphamide, bortezomib and dexamethasone | Other regimensa | ||
|---|---|---|---|---|---|---|
| A | Evaluable patients,n(%) | 472 (100) | 232 (49.2) | 86 (18.2) | 82 (17.4) | 72 (15.2) |
| B | Age at diagnosis, median (range in years) | 57 (29–71) | 56 (33–69) | 57 (34–69) | 61 (38–71) | 56 (29–71) |
| Male/female,n(%) | 279 (59)/193 (41) | 143 (62)/89 (38) | 49 (57)/37 (43) | 48 (58.5)/34 (41.5) | 39 (54)/33 (46) | |
| Multiple myeloma type,n(%) | ||||||
| Serum paraprotein IgG | 262 (55.5) | 127 (54.7) | 46 (53.5) | 55 (67) | 34 (47.2) | |
| Serum paraprotein non-IgG | 87 (18.4) | 39 (16.8) | 20 (23) | 12 (15) | 16 (22.2) | |
| Light chain only | 83 (17.6) | 41 (17.7) | 16 (19) | 15 (18) | 11 (15.3) | |
| Kappa | 55 (11.7) | 23 (10) | 10 (12) | 11 (13) | 11 (15.3) | |
| Lambda | 27 (5.7) | 17 (7.3) | 6 (7) | 4 (5) | – | |
| Not specified | 1 (0.2) | 1 (0.4) | – | – | – | |
| Non-secretory | 19 (4) | 14 (6) | 3 (3.5) | – | 2 (2.8) | |
| Plasma cell leukaemia | 5 (1.1) | 2 (0.9) | 1 (1) | – | 2 (2.8) | |
| POEMS | 1 (0.2) | – | – | – | 1 (1.4) | |
| Not available | 15 (3.2) | 9 (4) | – | – | 6 (8.3) | |
| Salmon–Durie Stage,n(%) | ||||||
| I | 50 (10.6) | 24 (10.3) | 7 (8) | 11 (13.4) | 8 (11) | |
| II | 45 (9.5) | 26 (11.2) | 6 (7) | 9 (11) | 4 (5.6) | |
| III | 350 (74.2) | 170 (73.3) | 73 (85) | 61 (74.4) | 46 (64) | |
| Not available | 27 (5.7) | 12 (5.2) | – | 1 (1.2) | 14 (19.4) | |
| International Staging System,n(%) | ||||||
| I | 98 (20.8) | 46 (19.8) | 23 (26.7) | 17 (21) | 12 (16.7) | |
| II | 118 (25) | 45 (19.4) | 28 (32.6) | 28 (34) | 17 (23.6) | |
| III | 107 (22.7) | 34 (14.7) | 30 (34.9) | 33 (40) | 10 (13.9) | |
| Not available | 149 (31.6) | 107 (46.1) | 5 (5.8) | 4 (5) | 33 (45.8) | |
Including: single agent dexamethasone (n=29); melphalan and prednisone (n=12); thalidomide and dexamethasone (n=6); lenalidomide and dexamethasone (n=3); bortezomib, dexamethasone, cisplatin, doxorubicin, CTX and etoposide (n=3); thalidomide, CTX and dexamethasone (n=1); CTX and dexamethasone (n=1); melphalan, prednisone and bortezomib (n=1); rituximab, adriamycin, CTX and prednisone (n=2); this also includes 4 patients who initially received melphalan and prednisone and were switched to VAD because of melphalan intolerance (n=1) or as an attempt to deepen response (n=3). In (A), the percentage of patients refers to all the studied patients (n=472). In (B), the percentage indicates the number of patients who received each induction regimen.
The overall response rate after induction was 77% (n=292) among the patients whose response data was known (n=379): 54 (14.2%) patients achieved CR, 65 (17.2%) achieved VGPR and 173 (45.7%) had a PR. The other patients were in SD (75, 20%) or PD (12, 3%) when they were seen for HSPC mobilization.
The ISS stage was not available for many patients diagnosed before 2005 (n=112) or who were referred from other centres (n=26), while the DS stage was unavailable in the medical records of early-diagnosed patients (n=15) and for some patients referred from outside TOH (n=12). The overall survival from the time of diagnosis and the time of aHSCT is presented in supplement Figure S2.
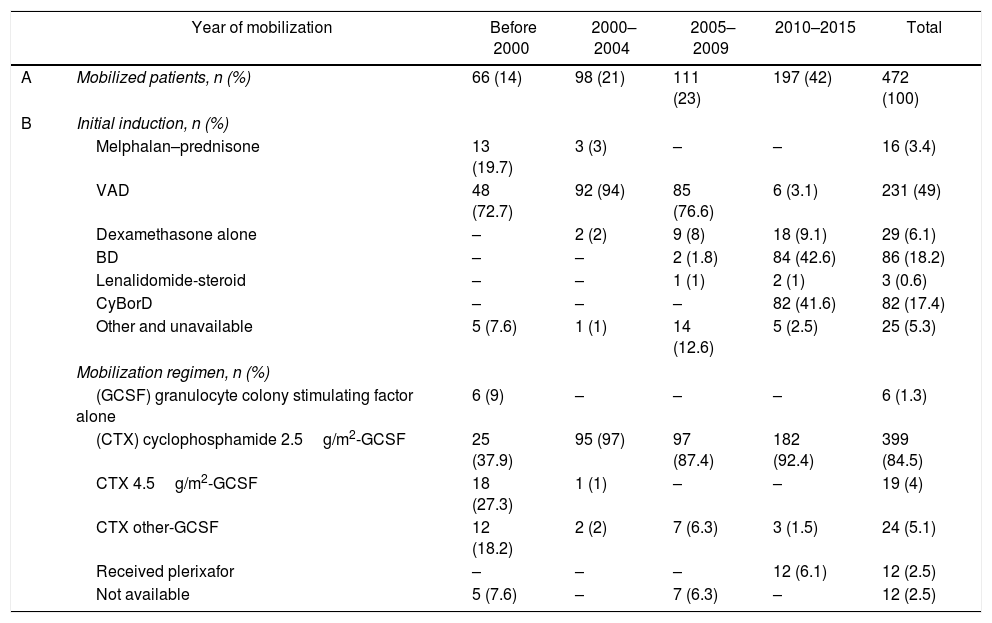
Remission inductionThe VAD, BD, and CyBorD regimens were the 3 most frequently used induction therapies for newly-diagnosed MM patients (Table 2) and reflected the evolving practice of the Myeloma Program at TOH throughout the study period. A minority of patients received other induction regimens (see Table 1). Information on the induction therapy was unavailable for 10 patients.
Myeloma therapy over time.
| Year of mobilization | Before 2000 | 2000–2004 | 2005–2009 | 2010–2015 | Total | |
|---|---|---|---|---|---|---|
| A | Mobilized patients, n (%) | 66 (14) | 98 (21) | 111 (23) | 197 (42) | 472 (100) |
| B | Initial induction, n (%) | |||||
| Melphalan–prednisone | 13 (19.7) | 3 (3) | – | – | 16 (3.4) | |
| VAD | 48 (72.7) | 92 (94) | 85 (76.6) | 6 (3.1) | 231 (49) | |
| Dexamethasone alone | – | 2 (2) | 9 (8) | 18 (9.1) | 29 (6.1) | |
| BD | – | – | 2 (1.8) | 84 (42.6) | 86 (18.2) | |
| Lenalidomide-steroid | – | – | 1 (1) | 2 (1) | 3 (0.6) | |
| CyBorD | – | – | – | 82 (41.6) | 82 (17.4) | |
| Other and unavailable | 5 (7.6) | 1 (1) | 14 (12.6) | 5 (2.5) | 25 (5.3) | |
| Mobilization regimen, n (%) | ||||||
| (GCSF) granulocyte colony stimulating factor alone | 6 (9) | – | – | – | 6 (1.3) | |
| (CTX) cyclophosphamide 2.5g/m2-GCSF | 25 (37.9) | 95 (97) | 97 (87.4) | 182 (92.4) | 399 (84.5) | |
| CTX 4.5g/m2-GCSF | 18 (27.3) | 1 (1) | – | – | 19 (4) | |
| CTX other-GCSF | 12 (18.2) | 2 (2) | 7 (6.3) | 3 (1.5) | 24 (5.1) | |
| Received plerixafor | – | – | – | 12 (6.1) | 12 (2.5) | |
| Not available | 5 (7.6) | – | 7 (6.3) | – | 12 (2.5) | |
Changes in remission induction and mobilization regimen given over time. In (A), the percentage of patients refers to all the studied patients (n=472). In (B), the percentage was calculated from the number of patients successfully mobilized in each period.
The VAD regimen was first used in March 1994 and remained the predominant induction treatment until 2009. Bortezomib was used in selected patients as first-line therapy beginning in March 2008. Beginning in February 2010, all patients received either BD or CyBorD as induction therapy, based on their physicians’ preference, while CyBorD has been the standard induction regimen since August 2013.
Stem-cell mobilization and collectionThe CTX-GCSF was the most frequent mobilization regimen used throughout the study period, with CTX doses ranging from 1.5g/m2 to 4.5g/m2 (Table 2). Plerixafor was first given in March 2012, and 11 of the 12 patients given plerixafor had received induction therapy with CyBorD.
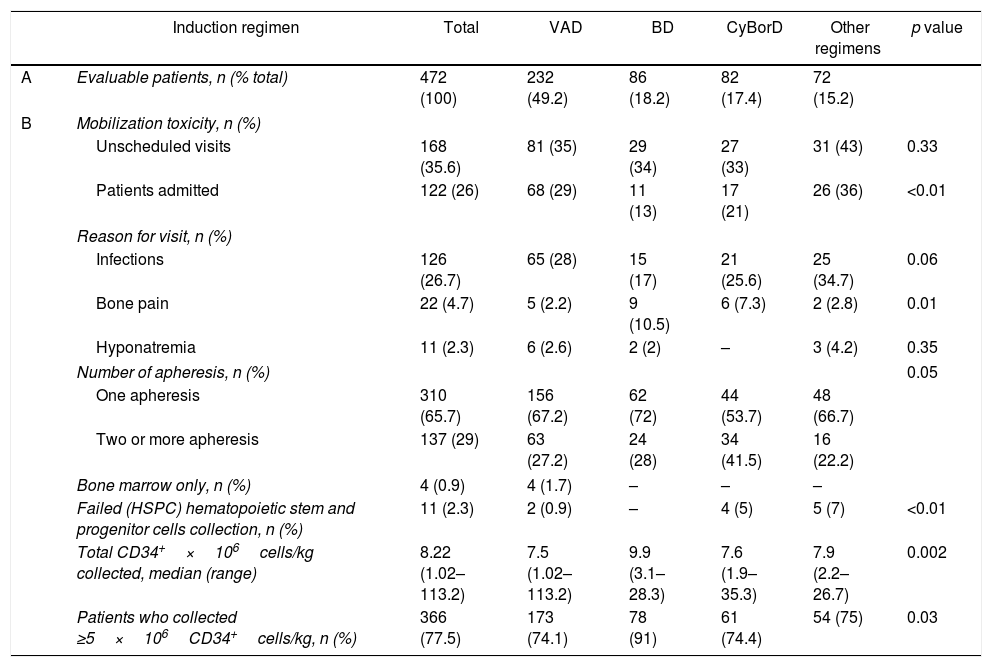
Toxic events, severe enough to warrant an unscheduled medical visit to the clinic or emergency room after mobilization, were not different between groups of patients who had received different induction regimens (p=0.33), but fewer hospital admissions were required by those who received BD (Table 3 and Table S2).
Mobilization and collection outcomes stratified by remission induction received.
| Induction regimen | Total | VAD | BD | CyBorD | Other regimens | p value | |
|---|---|---|---|---|---|---|---|
| A | Evaluable patients, n (% total) | 472 (100) | 232 (49.2) | 86 (18.2) | 82 (17.4) | 72 (15.2) | |
| B | Mobilization toxicity, n (%) | ||||||
| Unscheduled visits | 168 (35.6) | 81 (35) | 29 (34) | 27 (33) | 31 (43) | 0.33 | |
| Patients admitted | 122 (26) | 68 (29) | 11 (13) | 17 (21) | 26 (36) | <0.01 | |
| Reason for visit, n (%) | |||||||
| Infections | 126 (26.7) | 65 (28) | 15 (17) | 21 (25.6) | 25 (34.7) | 0.06 | |
| Bone pain | 22 (4.7) | 5 (2.2) | 9 (10.5) | 6 (7.3) | 2 (2.8) | 0.01 | |
| Hyponatremia | 11 (2.3) | 6 (2.6) | 2 (2) | – | 3 (4.2) | 0.35 | |
| Number of apheresis, n (%) | 0.05 | ||||||
| One apheresis | 310 (65.7) | 156 (67.2) | 62 (72) | 44 (53.7) | 48 (66.7) | ||
| Two or more apheresis | 137 (29) | 63 (27.2) | 24 (28) | 34 (41.5) | 16 (22.2) | ||
| Bone marrow only, n (%) | 4 (0.9) | 4 (1.7) | – | – | – | ||
| Failed (HSPC) hematopoietic stem and progenitor cells collection, n (%) | 11 (2.3) | 2 (0.9) | – | 4 (5) | 5 (7) | <0.01 | |
| Total CD34+×106cells/kg collected, median (range) | 8.22 (1.02–113.2) | 7.5 (1.02–113.2) | 9.9 (3.1–28.3) | 7.6 (1.9–35.3) | 7.9 (2.2–26.7) | 0.002 | |
| Patients who collected ≥5×106CD34+cells/kg, n (%) | 366 (77.5) | 173 (74.1) | 78 (91) | 61 (74.4) | 54 (75) | 0.03 | |
Mobilization toxicity and HSPC collection effectiveness were analyzed according to remission induction received. The collection of at least 5×106CD34+cells/kg was considered sufficient for two aHSCTs. In (A), the percentage of patients refers to all the studied patients (n=472). In (B), the percentage was calculated from the number of patients who received each induction regimen.
Infections were the most common adverse events after HSPC mobilization (Table 3) and trended towards a lower frequency in the cohort that had previously received BD induction. On the other hand, a greater proportion of patients experienced bone pain during the mobilization period, for those who had previously received BD and CyBorD (p=0.01).
Among those admitted to hospital, the median length of hospital stay was 3 days, irrespective of the remission induction given (p=0.86). Only one patient required ICU care after mobilization due to hyponatremia. Eleven patients mobilized with CTX-GCSF failed to collect sufficient HPSC to proceed with one aHSCT (Table 3), thus remaining 461 patients, who proceeded to a first-line aHSCT.
The increase in the proportion of patients achieving VGPR or better after mobilization with cyclophosphamide did not seem significant (31.4% following induction and 36.3% following mobilization, p=0.18).
Stem-cell transplant and engraftment characteristicsOf the 461 MM patients who successfully collected HSPC, 55 underwent aHSCT out of TOH and they were excluded from the engraftment analyses. The conditioning regimens of the analyzed patients were melphalan 140–200 (Mel140–200) (87.4%), melphalan, VP-16 and total-body irradiation (MelVPTBI) (11.6%), busulfan, cyclophosphamide and total-body irradiation (BuCyTBI) (0.7%), and busulfan and melphalan hydrochloride (BuMel) (0.3%).
Six (1.4%) of the 406 transplanted patients died before engraftment. Neutrophil engraftment occurred in a median of 12 days after aHSCT (range 8–65 days) for all cohorts (p=0.52). Platelet engraftment occurred in a median of 12 days after aHSCT (range 7–151 days) and it likewise did not differ between cohorts (p=0.71). The platelet count of 17 (4%) patients never dropped below 20×109/L, and these patients did not require platelet transfusion support.
The neutrophil or platelet counts were abnormal in 12% of the whole cohort at the first anniversary of the aHSCT. The 3 patients that experienced severe neutropenia or thrombocytopenia in the 1-year follow-up of the aHSCT had relapsed myeloma and were on salvage therapy (able 4). Moderate cytopenias at 1 year after the aHSCT were felt to be due to relapsed myeloma (n=14), infection (n=5), lenalidomide maintenance therapy (n=2), amegakaryocytic thrombocytopenia (n=1) and heparin-induced thrombocytopenia (n=1). The cause of cytopenias was unexplained in 23 (5.7%) patients.
DiscussionThis study describes the outcomes of a large cohort of consecutive myeloma patients undergoing HSPC mobilization and collection for a first aHSCT at a single tertiary care hospital for 2½ decades. It provides “real world” data on the changes in patient induction therapy over the years, reflecting the evolution of myeloma care outside of clinical trials and how it influenced HSPC collection outcomes. Specifically, we analyzed the disease control in our cohorts, the impact of remission induction on HSPC mobilization and collection, and the toxicity secondary to the therapy received.
The myeloma response was deepened as remission induction regimens evolved over time, and a significant decrease in hospitalization after HSPC mobilization was observed as remission induction switched from VAD to bortezomib-based regimens. Patients who received BD remission induction suffered the fewest serious toxicities following mobilization and had the best collections, whether measured by number of cells or number of apheresis to achieve a target collection, illustrating how the first therapy given to MM patients impacts the subsequent treatments.
Our experience agrees with other studies showing that remission induction regimens differentially influence HSPC collection. Bortezomib-containing remission induction therapy is not associated with a detrimental impact on HSPC collection.20,21 Silvennoinen et al.22,23 found that lenalidomide produced negative effects on CD34+ cell mobilization, as the number of CD34+ cells collected by those who received lenalidomide, bortezomib and dexamethasone was approximately 32% lower than BD-treated patients. In our practice, when CTX was added to a BD-based remission induction regimen, patients were more likely to require a second apheresis to reach the CD34+ collection target. In contrast, Benyamini et al.,24 was able to reach a CD34+ cells target with one collection in 87% of CyBorD-treated patients mobilized with CTX-GCSF, although with a similar rate of rehospitalisation was observed in our cohort. Pozotrigo et al. performed a multivariate analysis of a retrospective cohort of 317 patients mobilized with CTX-GCSF or GCSF alone and found that none of the induction agents, including thalidomide, lenalidomide, bortezomib and a group of miscellaneous regimens, was significantly associated with poor HSPC mobilization and collection.25
This “real world” study illustrates the advances in the care of MM by looking at consecutive myeloma patients from a BMT database containing 25 years of follow-up data. The CTX-GCSF remained the most common mobilization regimen over the study period, which facilitated the analyses of the impact of different remission induction therapies on the toxicity seen during HPSC mobilization and on the effectiveness of adequate HSPC graft collection. As a retrospective study, our analyses were challenged by historically unavailable data. The completeness of outcome data was biased towards the early cohorts which had the longest follow-up, but who had received older regimens, which are no longer used in most centres.
Changes in the choice of induction therapy of MM patients can alter the results of downstream procedures such as HSPC mobilization and collection. It is important that ongoing quality initiatives be put in place to monitor these outcomes to ensure that changes in clinical operations remain beneficial to patients. In an era of remission induction regimens that include novel agents and that result in improved disease responses, the role of CTX for HPSC mobilization should be revisited and less toxic mobilization alternatives could be considered.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the Ottawa Gatineau Multiple Myeloma Support Group, the Myeloma team and the BMT team for their great support towards our research.