The catastrophic antiphospholipid syndrome (CAPS) is an autoimmune disease associated with rapid failure of multiple organs and represents less than 1% of all cases of the antiphospholipid syndrome (APS). Antiphospholipid antibodies are present in approximately 9% of patients with pregnancy losses, 14% with stroke, 11% with myocardial infarction and 10% with deep vein thrombosis.1 The CAPS is considered a rare but devastating variant (30–50% mortality).2,3 It is diagnosed according to the Asherson criteria that include: an involvement of three or more organs, a rapid progression (simultaneously or in less than one week), a laboratory demonstration of antiphospholipid antibodies and histologic evidence of small vessel occlusion (may involve one or more sites and may be accompanied by vasculitis).
The American Society for Apheresis (ASFA) assigned category 2, grade 2C to the therapeutic plasma exchange (TPE) to treat the CAPS. Therefore, we face a disorder in which the TPE is considered a second-line treatment.4 Here we present a case of the CAPS whose initial presentation included multiple thromboses and which did not respond well to conventional treatment at the national reference Hospital Dos de Mayo, Lima, Peru, which was successfully treated with the TPE.
Case reportA 45-year-old diabetic patient, a farmer from Huancavelica, Peru presented a progressive condition which had been developing over the previous two months characterized by pain in the lower extremities and difficulty in walking. He had entered our emergency department with a 10-day history of symptoms of severe pain in the left leg and the inability to walk. The physical examination showed cyanosis and hypothermia in both knees, necrosis in the big toes, decreased femoral pulses, decreased popliteal impulses and the absence of a pulse in both feet. The neurological, respiratory and cardiovascular evaluations were normal.
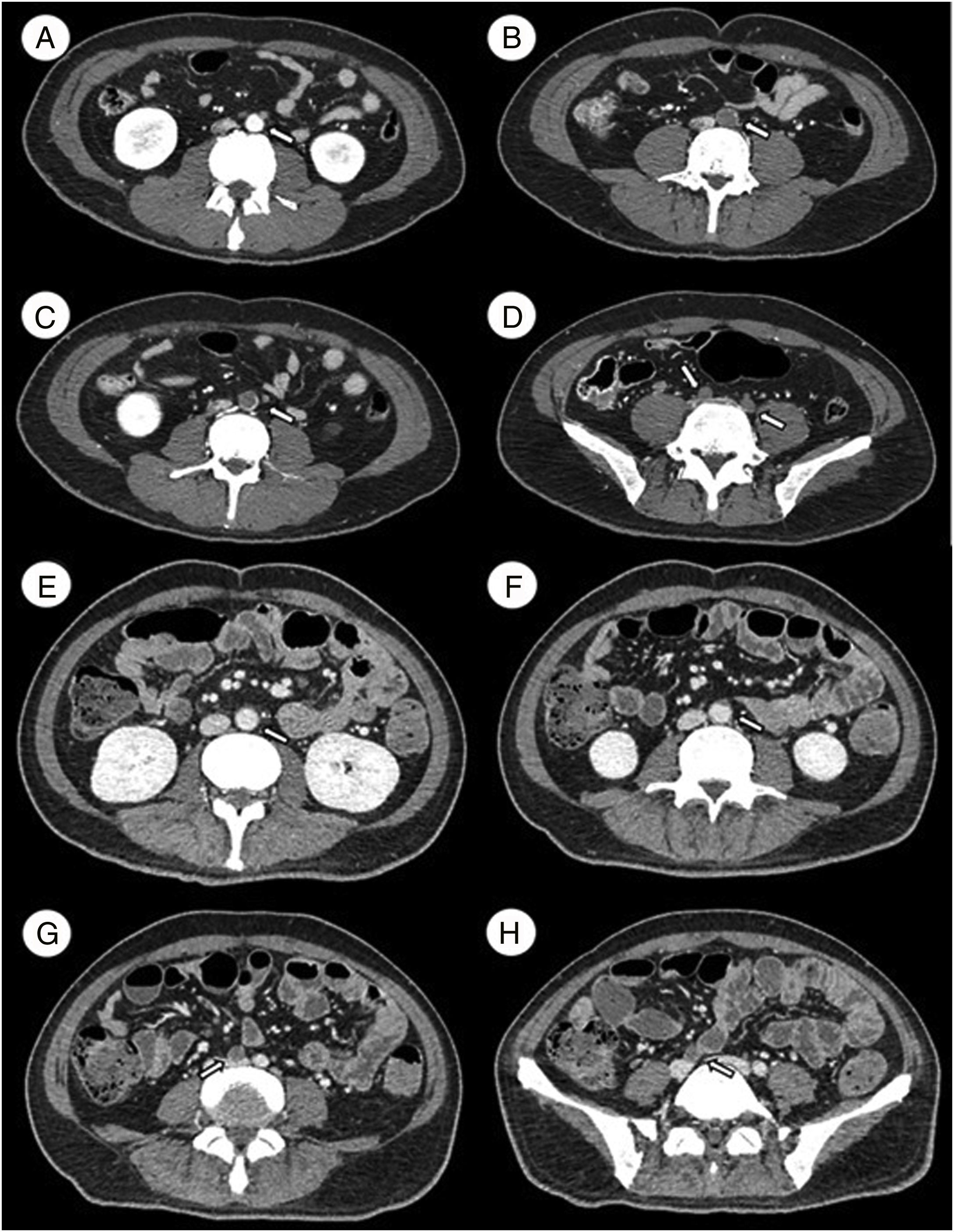
An angiotomography was performed immediately, which revealed a large arterial thrombus in the distal end of the abdominal aorta and both common iliac arteries, which caused the absence of blood flow in both legs (Figure 1A–D), so they were amputated. And a later study of the surgical sample confirmed the existence of the occlusive thrombus.
A–D Angiotomography on admission. A) L3. Permeable abdominal aorta. B and C) L4. An arterial thrombus that occludes the distal end of the abdominal aorta. D) L5. The arterial thrombus that occludes both common iliac arteries. E–H Angiotomography after treatment. E) L2. Permeable abdominal aorta. F) L3. The arterial thrombus that partially occludes the abdominal aorta. G and H) L4-L5. The arterial thrombus that occludes only the right iliac artery.
Days later, the patient developed symptoms related to an acute myocardial infarction. The electrocardiogram was compatible with an affectation of the inferior aspect of the myocardium and an echocardiographic study showed the presence of a thrombus in the left ventricle.
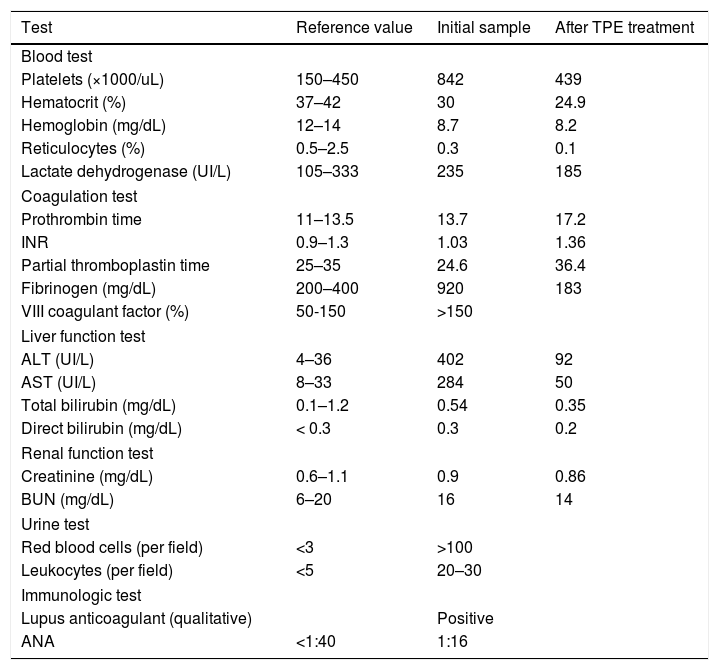
Based on these findings, thrombosis was suspected and treatment was started with a full dose of 80mg of enoxaparin (1mg/kg) every 12h and an initial dose of 300mg of clopidogrel, followed by a 75mg dose every 24h. Auxiliary examinations prior to the initiation of this treatment showed low hemoglobin values (8.7mg/dL) with a normal reticulocyte count (0.3%), thrombocytosis (842×10³/μL) and lactate dehydrogenase elevation (235 IU/L). In turn, the coagulation test showed a somewhat prolonged prothrombin time of 13.7s with a normal INR (international normalized ratio) value of 1.03, a partial thromboplastin time of 24.6s, a considerable increase in fibrinogen (920mg/dL) and a high value of coagulation factor VIII (>150%). The liver function test showed an increase in alanine-aminotransferase (ALT) and aspartate-aminotransferase (AST) at the values of 402 IU/L and 284 IU/L, respectively, normal values of total bilirubin (0.54mg/dL) and direct bilirubin (0.3mg/d:). The renal functional test showed the normal value of creatinine (0.9mg/d:) and blood urea nitrogen (BUN) of 16mg/dL. A urine test showed hematuria (>100 red blood cells per field) and leukocyturia (20–30 leukocytes×field). In immunological tests, a qualitative lupus anticoagulant test was positive in a sample taken after initiating the treatment with anticoagulants. The ANA test for lupus was negative (1:16). The anti-ß2-glycoprotein and anti-cardiolipin antibodies were not tested (Table 1).
Auxiliary examinations at the beginning and after the treatment with TPE.
| Test | Reference value | Initial sample | After TPE treatment |
|---|---|---|---|
| Blood test | |||
| Platelets (×1000/uL) | 150–450 | 842 | 439 |
| Hematocrit (%) | 37–42 | 30 | 24.9 |
| Hemoglobin (mg/dL) | 12–14 | 8.7 | 8.2 |
| Reticulocytes (%) | 0.5–2.5 | 0.3 | 0.1 |
| Lactate dehydrogenase (UI/L) | 105–333 | 235 | 185 |
| Coagulation test | |||
| Prothrombin time | 11–13.5 | 13.7 | 17.2 |
| INR | 0.9–1.3 | 1.03 | 1.36 |
| Partial thromboplastin time | 25–35 | 24.6 | 36.4 |
| Fibrinogen (mg/dL) | 200–400 | 920 | 183 |
| VIII coagulant factor (%) | 50-150 | >150 | |
| Liver function test | |||
| ALT (UI/L) | 4–36 | 402 | 92 |
| AST (UI/L) | 8–33 | 284 | 50 |
| Total bilirubin (mg/dL) | 0.1–1.2 | 0.54 | 0.35 |
| Direct bilirubin (mg/dL) | < 0.3 | 0.3 | 0.2 |
| Renal function test | |||
| Creatinine (mg/dL) | 0.6–1.1 | 0.9 | 0.86 |
| BUN (mg/dL) | 6–20 | 16 | 14 |
| Urine test | |||
| Red blood cells (per field) | <3 | >100 | |
| Leukocytes (per field) | <5 | 20–30 | |
| Immunologic test | |||
| Lupus anticoagulant (qualitative) | Positive | ||
| ANA | <1:40 | 1:16 | |
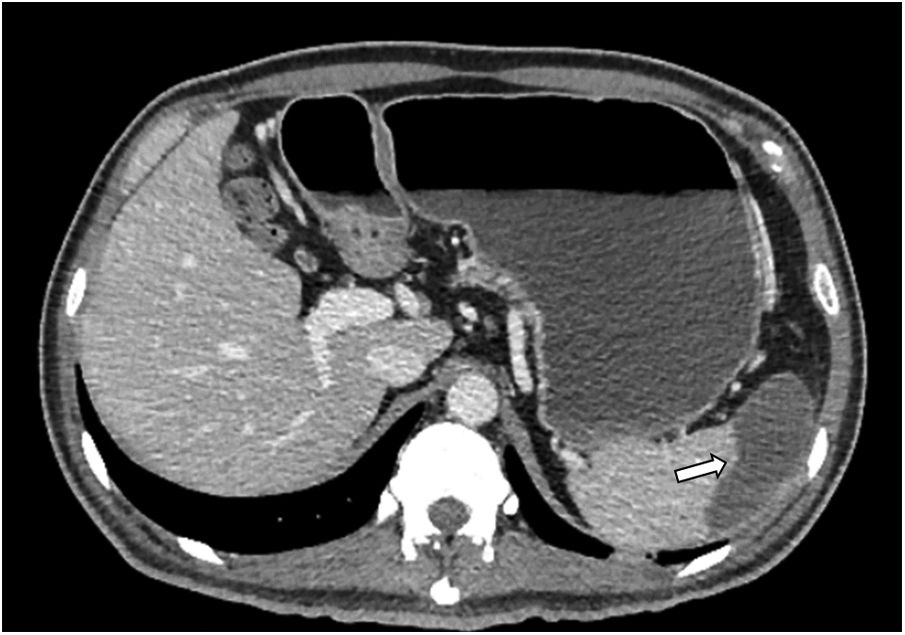
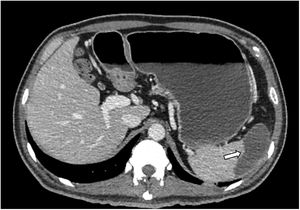
Based on the autoimmune etiology, eight doses of an attack with methylprednisolone were administered at a dose of 30mg/kg administered intravenously for one hour every 6h for two days. But a second angiotomography revealed the presence of a thrombus that occluded the distal part of the abdominal aorta, the inferior mesenteric artery and the common iliac artery. In addition, there was evidence of a splenic infarction of the upper pole (Figure 2). Due to this lack of response to conventional treatment and the diagnosis of an autoimmune disorder, it was decided to start the TPE.
Ten sessions of TPE were performed according to the following characteristics of the patient: 50kg of weight, 100cm of height and a hematocrit of 25%. The Spectra Optia apheresis system was used when exchanging 1.0 volume of plasma per procedure, using 5% albumin as a fluid replacement, without any relevant adverse event. The average plasma removal efficiency (PRE) of the device was 83.2% (minimum 82.7%, maximum 87.1%). A favorable response was observed at the end of the TPE procedures because we showed a favorable reduction in some parameters such as platelets (439×10³/μL), reticulocytes (0.1%), lactate dehydrogenase (185 IU/L), fibrinogen (183mg/dL), ALT (92 IU/L), AST (50 IU/L), total bilirubin (0.35mg/dL), direct bilirubin (0.2mg/dL), creatinine (0.86mg/dL) and BUN (14mg/dL). The hemoglobin (8.2mg/dL) did not show a significant decrease. The prothrombin time was extended to 17.2s with an INR of 1.36 and the partial thromboplastin time, to 36.4s. Finally, additional angiograms revealed a decrease in the size of the thrombus and the presence of blood perfusion (Figure 1E–H).
At the time of discharge, we found a patient with favorable evolution who showed stable vital functions, pain improvement, absence of angina, absence of dyspnea and ability to perform limited daily activities with support. The patient returned to his province and did not attend scheduled appointments for follow-up, so we cannot detail his subsequent evolution.
DiscussionBased on multiorganic alteration, rapid progression, positive results of antiphospholipid antibodies and anatomopathological evidence, a confirmatory diagnosis of CAPS was made.2 CAPS is a fatal variant of APS, with a prevalence of 1% in the population of patients with the latter. Given the low frequency of CAPS, our ability to systematically analyze and study it has been a challenge.5
The main organs involved are kidney (64%), lung (64%), brain (62%), heart (51%) and skin (50%). Our patient developed a myocardial infarction on the inferior anteroseptal side, renal insufficiency and splenic infarction, which is relatively rare (19%).5
The case reported in this study is a good example of a primary APS, which is reported in 40% of all APS cases. In addition, the CAPS, as the first manifestation of the sodium acetate-acetic acid-formalin fixative (SAF), has been reported in 46% of all cases. The triggers are infections, surgery, withdrawal of anticoagulation, medications, obstetric complications, neoplasia and lupus outbreak.6 In our patient, we could not identify a triggering factor.
In the differential diagnoses of the present clinical case, no clinical findings such as splenomegaly or lymphadenopathy were found that would support the diagnosis of malignant neoplasms (leukemia or myelodysplastic syndromes). Therefore, we did not see relevance in performing immunophenotyping tests. With respect to thrombotic microangiopathies (TMA), thrombosis was observed in large vessels (abdominal aortic artery and common iliac arteries), which distanced the diagnostic suspicion from a TMA, despite evidence of thrombocytopenia, anemia and elevated lactate dehydrogenase.
Given the known benefits of TPE in the treatment of microangiopathies, as well as the proven pathogenicity of antiphospholipid antibodies themselves, TPE has been proposed as a potential therapy for CAPS.7–9 Retrospective studies have shown a survival of 77.8% among patients treated with the triple therapy (anticoagulants, steroids and TPE), compared to 55.4% of patients who did not receive TPE.9 Patients who received this combination therapy were treated with TPE using fresh frozen plasma (FFP) as a replacement fluid, assuming that FFP may contain beneficial antithrombotic factors, so it was not clear whether the use of albumin instead of FFP as a replacement fluid would have similar benefits.10 Our patient presented a favorable response after treatment with TPE using 5% albumin as a replacement fluid, anticoagulants and steroids. However, the initiation of this treatment can be considered inadequate, as the CAPS had already produced a large systemic participation.
The absence of biomedical resources was, and will be, a very important limitation for a better approach to similar clinical cases. In turn, the same applies to the absence of standardized protocols to address thrombocytosis at our hospital, if the coordination between services that could have shortened the time in which the TPE was initiated has been delayed. On the other hand, it is the first CAPS report satisfactorily treated at a national reference hospital in an underdeveloped country such as Peru.
Conflicts of interestThe authors report not having any conflicts of interest. The patient gave his oral consent for the presentation of this report.
The authors are grateful for the external review and support in the writing of this article by Kelly Meza-Captcha.