Splenic hamartomas are benign and rare vascular tumors with no more than 200 cases reported in the literature since its initial description by Rokitansky in 1861.1–3 Of unknown pathogenesis, these tumors may consist of congenital developmental defects or acquired non-neoplastic malformations.4–6 Hamartomas are characterized by an abnormal mixture of native tissues that make up the affected organ.7 Splenic hamartomas (also called splenomas, nodular hyperplasia of the spleen, splenoadenomas, congenital tumor-like malformations, spleen with accessory spleen, or intrasplenic splenunculus) are frequently associated with benign hematologic diseases and hematologic malignancies.4 Although disseminated infections caused by fungi or mycobacteria may present as solid lesions in the spleen, the association between splenic hamartomas and infections has not been reported.8
The present report describes a patient with splenic lesions who was initially considered for the diagnosis of aggressive splenic lymphoma. Bone marrow aspirate and histological analysis of spleen and accessory spleen confirmed the diagnosis of splenic hamartoma associated with visceral leishmaniasis.
Case reportA previously healthy 65-year-old male retired farmer was referred to outpatient evaluation for pancytopenia. The patient reported fever (38°C), profuse night sweats, anorexia, abdominal distension, epigastric pain present for the previous 6 months, and weight loss (7% of the body weight) in the last 8 weeks. Initial physical examination revealed pallor, a slightly distended abdomen, and a palpable spleen 7cm below the left costal margin. Hepatomegaly and lymphadenopathy were not observed. The patient had resided in a non-endemic area for visceral leishmaniasis and did not report travelling to endemic areas.
Initial laboratory evaluation revealed: mild microcytic anemia (Hb 11.3g/dL; mean corpuscular volume 71.5fL), leukopenia (2.74×109/L), neutropenia (1.31×109/L) and thrombocytopenia (60×109/L). The following laboratory parameters were abnormal: serum lactate dehydrogenase (762 U/L; normal range: 135–225 U/L), C-reactive protein (15.2mg/dL; normal range: <0.5mg/dL), erythrocyte sedimentation rate (11mm), serum ferritin (6381ng/dL; normal range: 29–300ng/dL), aspartate aminotransferase (87.2U/L; normal range: <40U/L) and alanine aminotransferase (48.2U/L; normal range: <41U/L). Renal and thyroid functions were normal. Serologies for HIV, viral hepatitis (B and C), and syphilis were non-reactive. Serology for leishmaniasis was not performed. Serum protein electrophoresis revealed a monoclonal peak (0.64g/dL; IgG/lambda monoclonal protein defined by immunofixation of serum proteins) associated with a polyclonal increase in gammaglobulins. Blood and bone marrow cultures did not result in the growth of infectious agents.
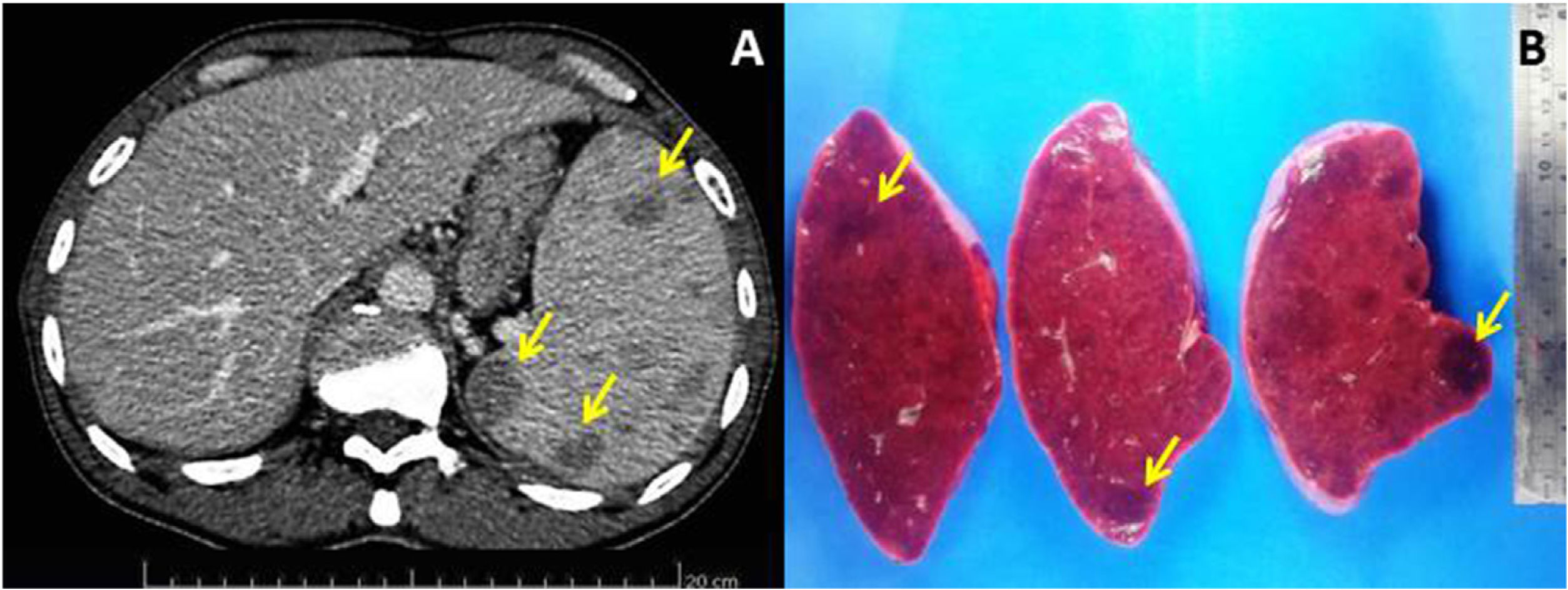
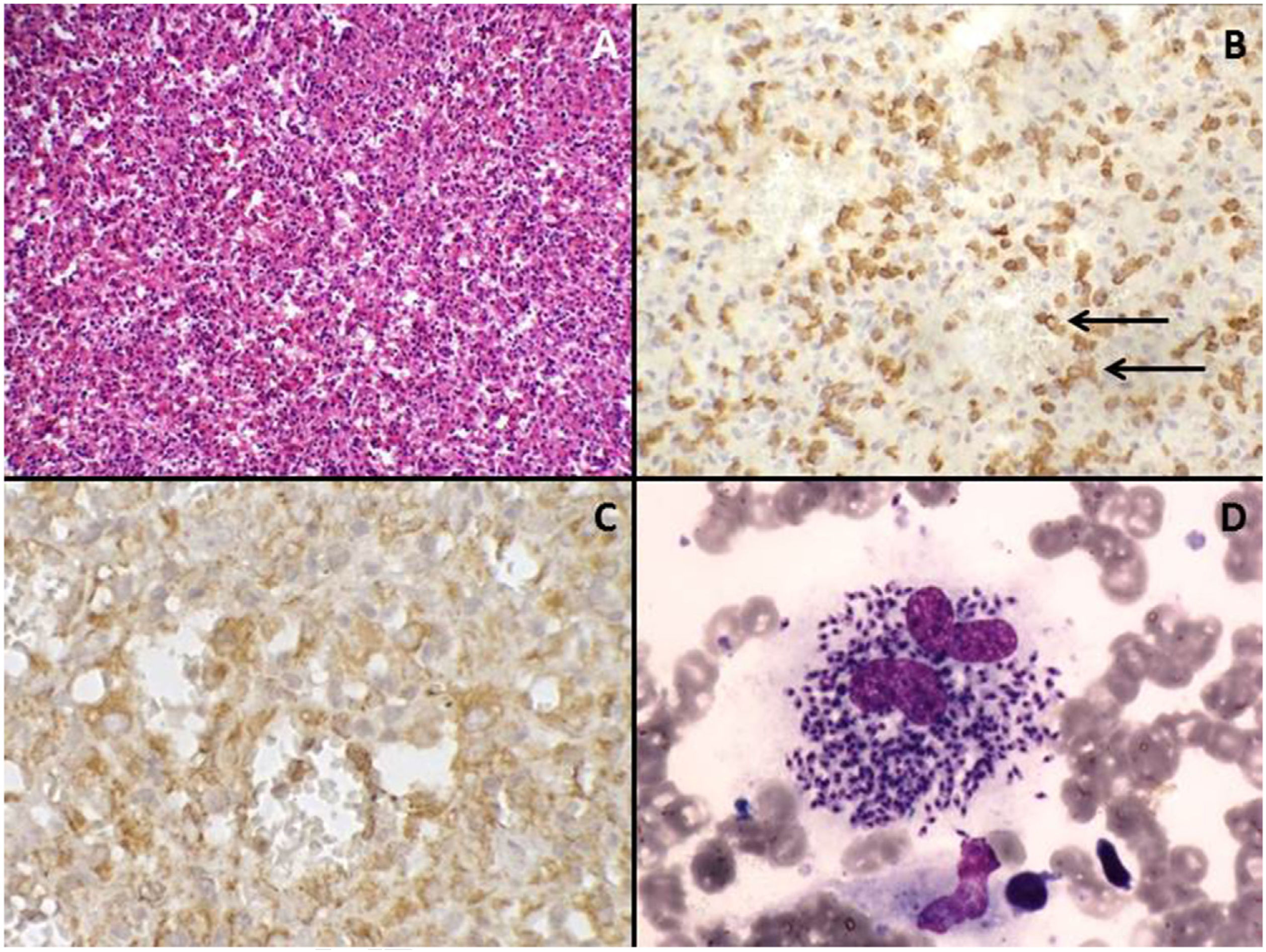
Bone marrow aspiration and biopsy ruled out the presence of infectious agents or hematological malignancies. Contrast-enhanced abdominal computed tomography scan identified that the spleen was increased in size (splenic index: 2210; normal up to 480) and contained multiple hypodense nodules of up to 2.5cm (Fig. 1A); moreover, the accessory spleen (3cm) was affected by hypodense nodules of up to 1cm. A chest computed tomography scan showed no lesions. Due to a suspected diagnosis of aggressive splenic lymphoma, a total splenectomy was performed 5 weeks after the first evaluation (Fig. 1B). Histological and immunohistochemical evaluation of the spleen and accessory spleen revealed the presence of a histiocyte-rich hamartoma (Fig. 2A–C); there was no evidence of malignancy or presence of infectious agents.
(A) The nodules are a disorganized red-pulp-like proliferation without lymph follicle atypia, mitosis, or necrosis. Hematoxylin-eosin stain, 200× magnification. (B) Sinusoids within the nodules are partly lined with CD8+ cells (arrows), 200× magnification. (C) Red-pulp-like nodule with CD68+ cells both dispersed as well as partly lining the sinusoids (histiocyte-rich splenic hamartoma), 400× magnification. (D) Numerous amastigotes forms of Leishmania ssp. in the cytoplasm of macrophages in bone marrow smear. Leishman staining, 1000× magnification.
In the post-splenectomy follow-up, there was the cessation of fever and night sweats. In addition, lower serum lactate dehydrogenase (317U/L), lower serum ferritin (1666ng/dL) level, and normal platelet and leukocyte counts were observed. However, there was recurrence of fever 24 days after splenectomy with persistent anemia (Hb 8.8g/dL). The patient was admitted for infection screening, antibiotic therapy, and further bone marrow analysis (cytology and culture for bacteria, fungi, and mycobacteria). Cytological analysis of the bone marrow revealed the presence of amastigote forms of Leishmania ssp. (Fig. 2D). Upon confirmation of the diagnosis of visceral leishmaniasis, the patient was treated with meglumine antimony, which was later replaced by liposomal amphotericin B because of side effects. The infection was controlled, and the patient recovered physically with a normalization of laboratory parameters. Thirty months after the splenectomy, the patient remains well and is asymptomatic.
Peripheral blood and bone marrow cultures did not result in the growth of Leishmania ssp. Retrospective cytological and histological analysis of bone marrow (study prior to splenectomy) and spleen did not reveal infectious forms of Leishmania ssp.
DiscussionBenign tumors of the spleen may present as cysts, hemangiomas or hamartomas.3 Splenic hamartomas can occur at any age with equal incidence in both sexes, being commonly detected incidentally or in autopsy studies.9 Its estimated incidence, based on autopsy studies, ranges between 0.024% and 0.13%, and based on splenectomy studies, ranges between 0.015% and 2.7%.3,5 Advances and broad access to imaging examinations have resulted in a higher detection of asymptomatic splenic hamartomas.3,6
Splenic hamartomas may present as single or multiple, well-defined, non-encapsulated lesions with compression of the adjacent normal splenic parenchyma.3,6 In addition, fibrosis or cysts may be found. Although the pathogenesis of splenic hamartomas is unclear, it may correspond to congenital malformations of the splenic red pulp or acquired malformations with abnormal and disorganized growth of the splenic red pulp.5,6 An analysis of 105 cases published up to 1991 found that 74% of the cases were acquired (26% congenital) and 56% were associated with abnormalities or hematological diseases.4 The acquired nature of splenic hamartomas is supported by its association with malignancies, especially hematological malignancies. Hamartoma should be considered a potential diagnosis when new splenic lesions are detected in patients with previously known malignancy.3,5
The symptoms of splenic hamartomas, when present, are nonspecific and include abdominal pain and distension, with clinical manifestations directly related to the tumor and spleen volumes.3 Women tend to have tumors of higher volume (hormonal stimulus) than men.7 Children tend to have more frequent clinical manifestations than adults.10 Cytopenias are infrequent and, when present, are directly associated with the spleen volume (hypersplenism).2 Unusual presentations such as splenic rupture have already been described.9
Advances in the imaging methods (Doppler ultrasound, computed tomography, and magnetic resonance imaging) have allowed for improved differentiation of several conditions affecting the spleen, including the diagnosis of high suspicion for hamartomas.3,6 Cytological analysis of suspected splenic lesions using fine needle aspiration has limitations, in that it does not allow for the architectural analysis of the lesion for a reliable diagnosis.3 As diagnosis without sampling of intact splenic tissue is challenging, partial or complete splenectomy by laparotomy or laparoscopy is usually required for diagnostic purpose.2,3,10 Although imaging studies may suggest the diagnosis of hamartoma, only the histological analysis, complemented by an immunohistochemical study, can confirm the diagnosis of hamartoma among the possible differential diagnoses and allows for the exclusion of malignancies.7
Histological features include disorganized vascular channels lined by plump endothelial cells, mixed with red pulp stroma, without white pulp or organized lymphoid tissue.5–7 Other components of the red pulp (macrophages, CD34+ capillaries and myoid cells) may be found in different proportions.7 Differential diagnosis includes other vascular tumors.6,9,10 Immunohistochemical stains may help in this distinction. Because hamartoma is of sinusoidal origin, cells show reactivity for T-lymphocytes and the endothelial cell markers. The main immunohistochemical feature of splenic hamartoma is the positivity for CD8 on lining cells of the vascular channels.6 Other findings include the positivity for CD31, CD34 (variable), factor VIII-related antigen and vimentin and the negativity for CD68 and CD21 on endothelial cells.5,6,10 As shown in the present case, CD68+ cells border sinusoids in the unusual histiocyte-rich variant of splenic hamartoma.7 Among the differential diagnoses, vascular tumors of the spleen (hemangiomas, lymphangiomas, hemangioendotheliomas, littoral cell angiomas, sclerosing angiomatoid nodular transformation of the spleen and angiosarcomas) should be considered.9 Other conditions that form solid lesions in the spleen can be considered as differential diagnoses, including sarcoidosis, mycobacterial infections, disseminated mycoses, metastases, lymphomas, and inflammatory myofibroblastic tumors.6,8
Visceral leishmaniasis is a chronic parasitic disease that mainly affects the lymph nodes, liver, spleen, and bone marrow. The immunological mechanisms have not yet been defined. The spleen, as an organ of the mononuclear phagocytic system, plays a unique role in the pathophysiology of the disease by producing cytokines, among other mechanisms. After an extensive review of the literature, this is the first report of an association between splenic hamartoma and visceral leishmaniasis. We believe that in the present case, the inflammatory state associated with chronic infection was the stimulus for the emergence and growth of tumor lesions. The infectious load (amastigote forms) was not high enough to allow for the diagnosis through direct detection in cytological and histological analyses of pre-splenectomy bone marrow samples. Splenectomy resulted in additional immunosuppression, which in turn led to an increase in infectious load, thereby resulting in the subsequent direct detection of amastigote forms of Leishmania ssp.
In view of the increasing detection of asymptomatic splenic lesions, a better understanding of several tumors affecting the spleen is essential. This progress will allow for differentiation between splenic lesions, ensuring that the diagnosis of malignancies is not delayed or missed. The pathogenesis of hamartomas has still not yet been elucidated; however, visceral leishmaniasis may be an acquired etiology of these tumors.
Conflicts of InterestThe authors declare no conflicts of interest.