The serological screening of blood donors has been instituted by the Brazilian Ministry of Health and is mandatory in the research on several diseases transmissible through blood transfusion. Blood banks need to establish a screening service capable of reducing associated transfusions risks.
ObjectiveThe objective of this study was to establish the prevalence of transfusion-transmissible infection markers in donors at a hemotherapy service located in southwest Bahia, Brazil.
MethodsA retrospective study was performed between 2010 and 2016. Variables, such as the characterization of donors who were suitable and unsuitable for donation (in clinical screening), stratification by gender and age group and unsuitable samples for reactive test results (in serological screening) by aspecific antibodies against hepatitis B virus (anti-HBc and HBsAg), hepatitis C virus (anti-HCV), human T-lymphotropic virus (anti-HTLV I/II), HIV virus (anti-HIV I/II), chagas disease, and syphilis markers, were evaluated.
ResultsCollected data showed that 3.13% of the donors were considered unsuitable for serological screening and that the prevalence of reactive test results was higher in donors aged between 30 and 39 years and in males. The means of the serological markers was 1.09% for syphilis reagents, 0.63% for anti-HIV I/II, 0.51% for anti-HBc and anti-HCV, 0.15% for HBsAg, 0.14% for HTLV I/II and 0.10% for Chagas disease.
ConclusionThese results reflect the importance of awareness campaigns on sexually transmitted diseases and transfusion safety measures taken by hemotherapy services.
A human blood transfusion is a therapeutic option to temporarily correct a red blood cell, platelet or clotting factor deficiency. In some clinical situations, transfusion represents the only way to save a life or to rapidly improve a serious illness.1 Transfusion is estimated in several clinical situations, such as accidents, large surgeries, chronic diseases, coagulation problems, hematological diseases and pregnancy complications. For human use, blood components are required to be safe. Therefore, many trials are performed to ensure that transfusion occurs with the fewest possible incidents.2
Hemotherapy services are committed to adopting pre-transfusion procedures aimed at an efficient and timely preparation of blood products with the lowest possible risk for recipients. Thus, donation follows rigorous selection criteria in the stages of donor selection and blood collection, fractionation, immunohematology and serology analyses, storage and distribution.3 The clinical screening of the donor cannot confer transfusion safety alone. There can be an omission of relevant facts, such as chronic asymptomatic disease and infections unknown by the donor. For this reason, serological screening with laboratory tests of high specificity and sensitivity is required to reduce the risk of transmitting infectious diseases.4
The Brazilian Ministry of Health declared as transfusion-transmissible diseases investigated by transfusion services: syphilis, Chagas disease, hepatitis B, hepatitis C, HIV I/II and HTLV I/II. Malaria and cytomegalovirus are investigated particularly in endemic regions and in specific cases. Hence, serological screening generally avoids the occurrence of possible failures in the clinical screening of transfusion-transmissible infections.5
Confirmatory or complementary tests are optional for hemotherapy services, but it is the responsibility of bank blood to guide the donor with altered exams, referring him or her to specific care services for confirmation of the diagnosis and therapeutic orientation. In the case of the confirmation of the examinations performed by the blood bank, it is the responsibility of this service to refer the patient to the proper clinical orientation.6 Information on the seroprevalence of infectious diseases transmitted by transfusion in hemotherapy services can serve as a basis for health actions aimed at preventing the transmission of these diseases. Therefore, this study aimed to verify the prevalence of infectious diseases in blood donors at a hemotherapy service in the city of Vitória da Conquista and its evolution over time.
MethodologyA semi-quantitative study with retrospective analysis was performed. It covered 34,404 blood donors who were attended to at the service from January 2010 to December 2016. The study was conducted at the Southwestern Hemotherapy Service (SHS), a private blood bank that meets the demand of private hospitals and the Brazilian Unified Health System, located in southwest Bahia, Brazil.
The data compilation was performed by the computer system at the service studied. The characterization of donors suitable and unsuitable for donation was determined by the results of clinical screening and stratification by gender and age group, unsuitable status being due to reactive test results for the following markers: anti-HBc, HBsAg, anti-HCV, anti-HIV I/II, anti-HTLV I/II, Chagas disease (Elisa, chemiluminescence) and syphilis [Venereal Disease Research Laboratory (VDRL)], Fluorescent Treponemal Antibody Absorption Test (FTA-Abs test) and chemiluminescence-based test (using treponemal antigens). The collected data were organized on spreadsheets and then analyzed in the Epi Info software, version 7.2.0.1. The project was approved by the Ethics Committee for Research on Human Beings at the Multidisciplinary Institute for Health, determination: 2,034,011; April 26, 2017. The research was developed within the ethical standards established in the Brazilian Ministry of Health resolution 466/2012.
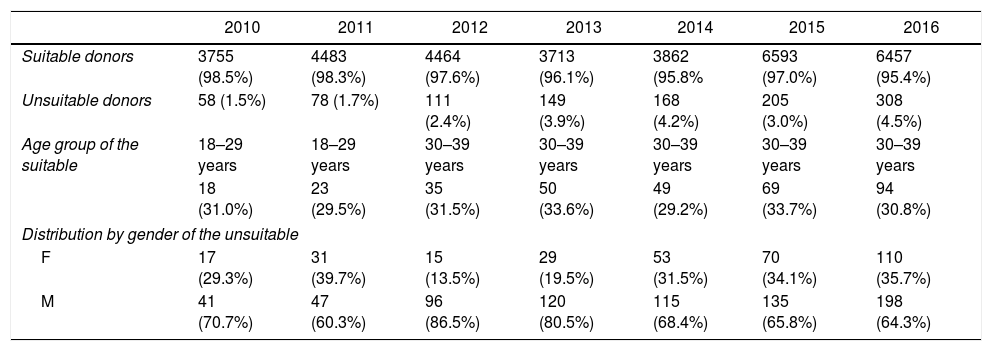
ResultsHemotherapy service studied attended 34,404 donors from January 2010 to December 2016. The highest annual occurrence of unsuitable donors in serological screening was in 2016 corresponding to 4.5%, and the lowest was in 2010, with 1.5% of donors (Table 1).
The characterization of the donors and the profile of unsuitable candidates attended at SHS from 2010 to 2016.
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | |
|---|---|---|---|---|---|---|---|
| Suitable donors | 3755 (98.5%) | 4483 (98.3%) | 4464 (97.6%) | 3713 (96.1%) | 3862 (95.8% | 6593 (97.0%) | 6457 (95.4%) |
| Unsuitable donors | 58 (1.5%) | 78 (1.7%) | 111 (2.4%) | 149 (3.9%) | 168 (4.2%) | 205 (3.0%) | 308 (4.5%) |
| Age group of the suitable | 18–29 years | 18–29 years | 30–39 years | 30–39 years | 30–39 years | 30–39 years | 30–39 years |
| 18 (31.0%) | 23 (29.5%) | 35 (31.5%) | 50 (33.6%) | 49 (29.2%) | 69 (33.7%) | 94 (30.8%) | |
| Distribution by gender of the unsuitable | |||||||
| F | 17 (29.3%) | 31 (39.7%) | 15 (13.5%) | 29 (19.5%) | 53 (31.5%) | 70 (34.1%) | 110 (35.7%) |
| M | 41 (70.7%) | 47 (60.3%) | 96 (86.5%) | 120 (80.5%) | 115 (68.4%) | 135 (65.8%) | 198 (64.3%) |
Abbreviations F: female; M: male.
Serological tests indicated 1077 unsuitable donors in serological screening. In all the years studied, there was a higher prevalence of reactive tests among men. Regarding the age group, the highest prevalence was of donors aged between 30 and 39 years, except in 2010 and 2011; in these years the prevalent age range was 18 to 29 years, corresponding to 31.03% and 29.49%, respectively.
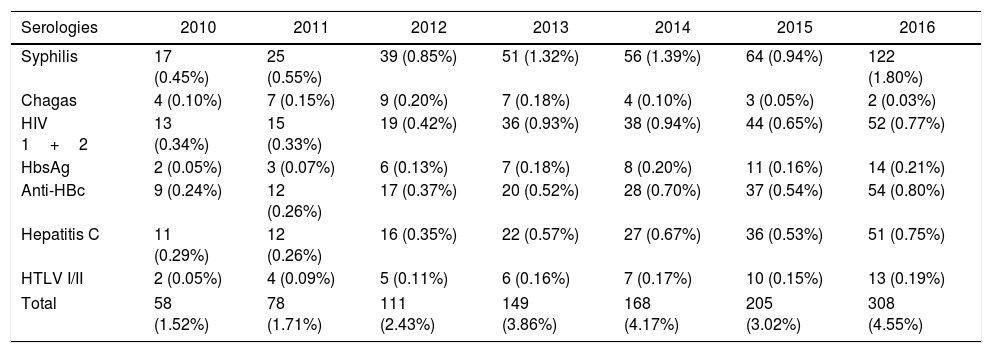
The annual assessment of the serological screening of blood donors demonstrated stability in all the years assessed, with the smallest overall prevalence of 1.5% in 2010 and the highest in 2016, of 4.5% (Table 2). The mean of the serological markers from 2010 to 2016 was 1.09% for syphilis reagent samples, 0.63% for anti-HIV-1/2, 0.51% for anti-HBC and anti-HCV, 0.15% for HBsAg, 0.14% for HTLV-I/II, and 0.10% for Chagas disease. From January 2010 to December 2016, a progressive increase in the reactive test result rate was observed. The serological marker for syphilis (Treponema pallidum) stands out with a high prevalence in 2016 (1.8%); in the same year, the lowest prevalence was the serology for Chagas disease, with two positive cases (0.03%).
Annual distribution of reactive test result for transfusion-transmitted diseases at SHS, from 2010 to 2016.
| Serologies | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|---|---|
| Syphilis | 17 (0.45%) | 25 (0.55%) | 39 (0.85%) | 51 (1.32%) | 56 (1.39%) | 64 (0.94%) | 122 (1.80%) |
| Chagas | 4 (0.10%) | 7 (0.15%) | 9 (0.20%) | 7 (0.18%) | 4 (0.10%) | 3 (0.05%) | 2 (0.03%) |
| HIV 1+2 | 13 (0.34%) | 15 (0.33%) | 19 (0.42%) | 36 (0.93%) | 38 (0.94%) | 44 (0.65%) | 52 (0.77%) |
| HbsAg | 2 (0.05%) | 3 (0.07%) | 6 (0.13%) | 7 (0.18%) | 8 (0.20%) | 11 (0.16%) | 14 (0.21%) |
| Anti-HBc | 9 (0.24%) | 12 (0.26%) | 17 (0.37%) | 20 (0.52%) | 28 (0.70%) | 37 (0.54%) | 54 (0.80%) |
| Hepatitis C | 11 (0.29%) | 12 (0.26%) | 16 (0.35%) | 22 (0.57%) | 27 (0.67%) | 36 (0.53%) | 51 (0.75%) |
| HTLV I/II | 2 (0.05%) | 4 (0.09%) | 5 (0.11%) | 6 (0.16%) | 7 (0.17%) | 10 (0.15%) | 13 (0.19%) |
| Total | 58 (1.52%) | 78 (1.71%) | 111 (2.43%) | 149 (3.86%) | 168 (4.17%) | 205 (3.02%) | 308 (4.55%) |
Important Considerations: During the studied period different methodologies were employed: Syphilis (VDRL, FTA-Abs, Chemiluminescence); Chagas and HTLV-I/II (Elisa 3rd generation and Chemiluminescence); Hepatitis B – HbsAg and Anti-HBc (Elisa 3rd generation, Chemiluminescence, NAT HBV); Hepatitis C – Anti-HBV (Elisa 3rd generation, Chemiluminescence, Electrochemiluminescence, NAT HBC); HIV-I/II (Elisa 3rd generation, Chemiluminescence, Electrochemiluminescence, NAT HIV).
In 2011, HbsAg had the lowest prevalence (0.07%). In 2012 and 2013, anti-HTLV accounted for 0.11% and 0.16%, respectively. Serologies mentioned were performed by the chemiluminescence method. In 2010, the markers that presented the lowest prevalence were HbsAg and HLTV, both presenting two cases (0.05%). In other years, there were no notable differences in the prevalence of different serological markers (Table 2).
DiscussionThe mean serological prevalence was 3.13% in the present study. Ramos et al.7 registered a prevalence of 7.58% in a study which evaluated blood donors from Campo Mourão, Paraná, Brazil. This difference can be due to the epidemiological characteristics of each region. A study conducted by Rodriguez et al.8 in Caxias do Sul, Rio Grande do Sul, Brazil, demonstrated a 2.5% unsuitable serological prevalence (due to reactive test results). An unsuitable serological prevalence of 3.99% was found in Criciúma, Santa Catarina, Brazil, by Oliveira et al.9 reflecting results close to those found in the present study.
In this study, the unsuitable serological patients were predominantly male (69.82%) and within the age range of 30–39 years. Rohr et al.10 and Tovo et al.11 also showed higher unsuitable serological prevalence in donors aged 30 years or older. In Ethiopia, most of the blood donors were in the age range of 26–35 years.12 However, our data, as well as that of others previously published in different countries, differ from the figures announced by the World Health Organization (WHO), which reported that 45% of donors were aged 25 or less.13 Thus, the need for awareness in all age groups is evident. According to Patavino,14 men report more sexual partners compared to women in the pre-donation interview, which assists in the comprehension of the greater serological inadequacy in male donors.7 Colli et al.15 emphasize, especially in the age group cited (30 years or older), the greatest number of unsuitable serological patients is due to sexual behavior and lifestyle.
Syphilis, an infectious disease of chronic progression, infects several systems. The disease is caused by the bacterium T. pallidum and transmitted mainly by sexual contact, blood transfusion and through the placenta.16 Data from Boff et al.17 and Oliveira et al.18 showed positive syphilis rates of 0.3% and 2.1%, respectively, differing from the results found in the present study (mean prevalence of 1.09%). Differences in prevalence can be related to the different epidemiological characteristics of each region and different detection methods used. Three detection methods were used from 2010 to 2016: the VDRL test, FTA-Abs test, and chemiluminescence-based test. The VDRL test has high sensitivity and low specificity. The FTA-ABS test shows a specificity between 96% and 99%. The chemiluminescence-based test presents a sensitivity of 89% and specificity of 99%.19 In this study, there was a higher prevalence of positive serology for syphilis among the markers studied. However, the prevalence had been increasing over the years. Thus, it is possible to associate the growth of positive cases over the years with the increase of the specificity of the exams performed in the hemotherapy service. The first one used was the VRDL test (2010–2014), followed by the FTA-ABS test (in 2015) and finally the chemiluminescence method (in 2016). Municipalities in the northeastern region of Brazil have a high prevalence of syphilis.20 In general, the results presented in this study point to the need for strategic actions aimed at reducing cases of syphilis in areas of high prevalence.
The marker for the human immunodeficiency virus (HIV), which causes AIDS (Acquired Immunodeficiency Syndrome), is of utmost importance in the screening of blood banks. Because of some genetic modifications, there are two types of HIV: HIV-I and HIV-II. In Brazil, HIV-I predominates, with HIV-II infection being uncommon. Regarding the seroprevalence for HIV-I/II, an average index of 0.63% was observed in the period studied, higher than the study by Lauermann et al.,21 which presented the prevalence index of 0.12%. Nevertheless, it is estimated that the number of HIV positive individuals in Brazil is around 0.6% of the general population, corresponding to the one observed in this study. In Goiânia, Goiás, Brazil, a study by Gonçalves et al.22 reported the prevalence of 0.8% for HIV, resembling that of this study.
Two serological markers are used for the hepatitis B virus (HBV): the HBs antigen (HBsAg) and the anti-HBc antibody. The HBsAg is a serological marker for hepatitis B that indicates acute or chronic infection. The anti-HBc, in turn, is a detectable marker during all the stages of the hepatitis B virus infection, except at the early stage of viral exposure. In the present study, the frequency of these markers was 0.66%, in the period studied and 2016 was the year of greatest prevalence (1.01%), thus representing the third most prevalent infectious disease in this study. Valente et al.2 presented in their studies an index of positivity for the upper HBV of 9.3%. A study by Alves et al.23 in Uberaba, Minas Gerais, Brazil found a prevalence of 5.6%, a higher value than that found in this study. However, Alves et al.23 pointed out that the prevalence of unsuitable donors for the HBV infection is higher in the state of São Paulo, Brazil, increasing to the northwest, being consequently lower in the region where the study was conducted.
The hepatitis C virus (HCV) has anti-HCV as its main serological marker. In the present study, the frequency of this marker represented the fourth cause of unsuitable serological screening, corresponding to 0.51%. This prevalence is higher than that of Salles et al.,6 which presented a positivity of 0.21%. However, data from this study resemble those described by Josahkian et al.24 in a study performed at a blood bank in Uberaba, Minas Gerais, Brazil, where the index was 0.4%. Intravenous illicit drug use is the factor most directly associated with the transmission of the disease and has been reported in HCV seroprevalence studies among blood donors, although it is a factor that makes the donor candidate already unsuitable in the clinical screening.25
Human T-lymphotropic virus type I and II (HTLV-I/II) was the first human retrovirus registered, due to its considerable worldwide prevalence. Globally, it is estimated between 10 and 20 million people are infected with HTLV-I.5 However, most of these people remain asymptomatic. Oliveira et al.26 reported a prevalence of HTLV-I/II of 0.2% in the Piauí, Brazil study, resembling the results found in this study (0.14%). In Brazil, the prevalence of seropositivity for HTLV-I/II is 0.8%.5
Chagas disease is a chronic systemic infection whose etiological agent is the protozoan Trypanosoma cruzi. The seroprevalence for Chagas disease in this study (0.10%) was lower than that of the study conducted in Porto Alegre, Rio Grande do Sul, Brazil by Fitarelli et al.27 In a study by Silva et al.28 performed at all the blood centers in Brazil, the prevalence for Chagas disease was 0.20%. Some studies have indicated a drop in the rates of disease positivity in some regions of Brazil, but continuous prevention measures are still necessary. The obligatory serological screening in hemotherapy services is an important factor contributing to the control of the disease.
The seroprevalence in the present study is close to that found by other studies also carried out in Brazil. However, several other countries have conducted seroprevalence studies of infectious diseases transmissible by blood transfusion, and the rates found varied widely among them. The seroprevalence of anti-HBsAg, anti-HCV, anti-HIV, and syphilis were respectively 10.9%, 0.4%, 0.1%, and 0.1% in Ethiopia.12 In India the seroprevalence for HIV, HBV, HCV, and infections by T. pallidum was 0.04%, 1.14%, 0.022% and 0.0006%, respectively.29 In Ghana, the seroprevalence rates of 3.8%, 0.7%, 8.4%, and 13.5% were obtained, respectively, for ant-HIV, ant-HTLV, ant-HCV, and T. pallidum.30 The difference in prevalence may be due to differences in the health system in the different scenarios of the study, as well as to the variable magnitude of risk factors for contracting transfusion-transmissible infections in various contexts.12 All of these studies contribute indirectly in the prevention of the transfusion of unsafe blood.
Clinical and serological screening is unquestionable, but Patavino14 emphasizes that blood banks should conduct clinical screening in a rational way because rigid clinical screening can exclude healthy donors and compromise the stockpiles of the blood banks. On the other hand, flexible clinical screening can harm receptors if there is no guarantee of good blood quality and absence of pathogens.
ConclusionThe prevalence of unsuitable donors in the serological screening of the present study was 3.13%, resembling the data published on the epidemiological profile of infectious diseases in Brazil. There was little growth in the general serological inability over the years, except for Chagas disease. The prevalence of each serological marker separately suggests the development of awareness-raising policies in the region studied. This measure can contribute to the reduction of the prevalence of markers and increase the safety of blood transfusions.
Meeting of ethical standardsThe project was approved by Ethics Committee for Research on Human Beings of Multidisciplinary Institute for Health (CEP-SERES HUMANOS IMS/CAT-UFBA), opinion: 2,034,011; April 26, 2017. The research was developed within the ethical standards set forth in resolution 466/2012, Brazilian Ministry of Health.
Conflicts of interestThe authors declare no conflicts of interest.